Feeding Habits of Wisconsin's Predominant Lotic Plecoptera, Ephemeroptera, and Trichoptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

Monte L. Bean Life Science Museum Brigham Young University Provo, Utah 84602 PBRIA a Newsletter for Plecopterologists
No. 10 1990/1991 Monte L. Bean Life Science Museum Brigham Young University Provo, Utah 84602 PBRIA A Newsletter for Plecopterologists EDITORS: Richard W, Baumann Monte L. Bean Life Science Museum Brigham Young University Provo, Utah 84602 Peter Zwick Limnologische Flußstation Max-Planck-Institut für Limnologie, Postfach 260, D-6407, Schlitz, West Germany EDITORIAL ASSISTANT: Bonnie Snow REPORT 3rd N orth A merican Stonefly S ymposium Boris Kondratieff hosted an enthusiastic group of plecopterologists in Fort Collins, Colorado during May 17-19, 1991. More than 30 papers and posters were presented and much fruitful discussion occurred. An enjoyable field trip to the Colorado Rockies took place on Sunday, May 19th, and the weather was excellent. Boris was such a good host that it was difficult to leave, but many participants traveled to Santa Fe, New Mexico to attend the annual meetings of the North American Benthological Society. Bill Stark gave us a way to remember this meeting by producing a T-shirt with a unique “Spirit Fly” design. ANNOUNCEMENT 11th International Stonefly Symposium Stan Szczytko has planned and organized an excellent symposium that will be held at the Tree Haven Biological Station, University of Wisconsin in Tomahawk, Wisconsin, USA. The registration cost of $300 includes lodging, meals, field trip and a T- Shirt. This is a real bargain so hopefully many colleagues and friends will come and participate in the symposium August 17-20, 1992. Stan has promised good weather and good friends even though he will not guarantee that stonefly adults will be collected during the field trip. Printed August 1992 1 OBITUARIES RODNEY L. -

Orden TRICHOPTERA Manual
Revista IDE@ - SEA, nº 64 (30-06-2015): 1–21. ISSN 2386-7183 1 Ibero Diversidad Entomológica @ccesible www.sea-entomologia.org/IDE@ Clase: Insecta Orden TRICHOPTERA Manual CLASE INSECTA Orden Trichoptera Carmen Zamora-Muñoz1, Marta Sáinz-Bariáin1 & Núria Bonada2 1 Departamento de Zoología. Facultad de Ciencias. Universidad de Granada. Campus de Fuentenueva, 18071 Granada (España). [email protected] 2 Grup de Recerca Freshwater Ecology and Management (FEM), Departament d’Ecologia, Facultat de Biologia, Universitat de Barcelona (UB), Diagonal 643, 08028 Barcelona, Catalonia (España). Imagen superior: Óleo con larva y adulto de tricóptero. Autora: Ana Sánz. 1. Breve definición del grupo y principales caracteres diagnósticos Los tricópteros o frigáneas (Trichoptera, del griego trichos, "pelo" y pteron, "ala") son artrópodos de la Clase Insecta cuyos adultos portan alas cubiertas de pilosidad. Casi todas sus especies dependen del medio acuático para su desarrollo. La mayoría habitan en ríos y arroyos de aguas limpias y bien oxigena- das, aunque también se pueden encontrar en ambientes lénticos, terrestres e incluso marinos. Forman un grupo natural y están cercanamente emparentados con las mariposas y polillas (Lepi- doptera), que tienen escamas en sus alas y, como ellos, son capaces de producir seda. Ambos forman el superorden Amphiesmenoptera. De hecho, el grupo es sobre todo conocido por la habilidad de sus larvas para fabricar, con seda y diversos materiales, una gran variedad de construcciones como estuches portáti- les (Figura 1), refugios fijos, redes para la recogida de alimento y galerías, por lo que también se les ha denominado “arquitectos subacuáticos” (Mackay & Wiggins, 1979; Wiggins, 2004). Aunque para la cons- trucción de los estuches los tricópteros utilizan el material disponible en el lecho del río, el tipo y disposi- ción de las piezas que usan suele tener un marcado carácter filogenético (https://www.youtube.com/ watch?v=3vr6Z54LJtM&spfreload=10). -

(Contd.) David DUDGEON Dr., Lecturer in Zoology Department Of
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Trichoptera Newsletter Jahr/Year: 1982 Band/Volume: 09 Autor(en)/Author(s): Anonym Artikel/Article: List of research workers (contd.) 9-11 © Hans Malicky/Austria; download- 9 unter- www.biologiezentrum.at LIST OP RESEARCH WORKERS (contd.) David DUDGEON Dr., Lecturer in Zoology Department of Zoology, University of Hong Kong, HONG KONG Present interests: Ecology, of stream and river dwelling Tricho- ptera in HongKongj ecological studies of fresh waters (lentie and lotie) in HongKong, freshwater macroinvertebrate ecology. Investigantion areas Asia. Willing to identify material for other workers: Yes, Asian material (larvae only). Information wanted: Any information dealing with Asian Tricho- ptera (and other freshwater insects). Other activities and interests: Freshwater molluscs, biology of decomposition, land-water interactive systems. Sue ELDIN, Miss M.Sc, research (towards Ph.D«,) Dept of Zoology, Chelsea College, University of London, Hortensia Road, London SW1O, England. Present interests: Agapetus fuscipes, Limnephilus lunatus, L.extricatus, Agraylea multipunctata, Hydroptila vectis and several others. Rearing of Trichoptera, distribution of Trichoptera along a tributary of the River Medway. Investigation" area: South-East England (partie.R.Medway,Kent). information wanted: Any information on light trap catches/lab rearing of any groups, pollution studies involving caddis. Other activities and interests: General aquatic entomology, limnology. Porntip CHMTARAMONGKOL, Miss M.Sc, University lecturer c/o Biologische Station Lunz, A - 3293 Lunz, Austria. Present interests:Taxonomy and ecological significance of all groups of caddisflies. Establishing a system of environ- ment indicators (doctoral thesis). Investigation area: Southern Asia. Material wanted: Any adult caddisflies from Southern Asia, in particular light trap material (including by-catches). -

List of Animal Species with Ranks October 2017
Washington Natural Heritage Program List of Animal Species with Ranks October 2017 The following list of animals known from Washington is complete for resident and transient vertebrates and several groups of invertebrates, including odonates, branchipods, tiger beetles, butterflies, gastropods, freshwater bivalves and bumble bees. Some species from other groups are included, especially where there are conservation concerns. Among these are the Palouse giant earthworm, a few moths and some of our mayflies and grasshoppers. Currently 857 vertebrate and 1,100 invertebrate taxa are included. Conservation status, in the form of range-wide, national and state ranks are assigned to each taxon. Information on species range and distribution, number of individuals, population trends and threats is collected into a ranking form, analyzed, and used to assign ranks. Ranks are updated periodically, as new information is collected. We welcome new information for any species on our list. Common Name Scientific Name Class Global Rank State Rank State Status Federal Status Northwestern Salamander Ambystoma gracile Amphibia G5 S5 Long-toed Salamander Ambystoma macrodactylum Amphibia G5 S5 Tiger Salamander Ambystoma tigrinum Amphibia G5 S3 Ensatina Ensatina eschscholtzii Amphibia G5 S5 Dunn's Salamander Plethodon dunni Amphibia G4 S3 C Larch Mountain Salamander Plethodon larselli Amphibia G3 S3 S Van Dyke's Salamander Plethodon vandykei Amphibia G3 S3 C Western Red-backed Salamander Plethodon vehiculum Amphibia G5 S5 Rough-skinned Newt Taricha granulosa -

Diverse Allochthonous Resource Quality Effects on Headwater Stream Communities Through Insect-Microbe Interactions
DIVERSE ALLOCHTHONOUS RESOURCE QUALITY EFFECTS ON HEADWATER STREAM COMMUNITIES THROUGH INSECT-MICROBE INTERACTIONS By Courtney Larson A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Entomology—Doctor of Philosophy Ecology, Evolutionary Biology and Behavior—Dual Major 2020 ABSTRACT DIVERSE ALLOCHTHONOUS RESOURCE QUALITY EFFECTS ON HEADWATER STREAM COMMUNITIES THROUGH INSECT-MICROBE INTERACTIONS By Courtney Larson Freshwater resources are vital to environmental sustainability and human health; yet, they are inundated by multiple stressors, leaving aquatic communities to face unknown consequences. Headwater streams are highly reliant on allochthonous sources of energy. Riparian trees shade the stream, limiting primary production, causing macroinvertebrates to consume an alternative food source. Traditionally, leaf litter fallen from riparian trees is the primary allochthonous resource, but other sources, such as salmon carrion associated with annual salmon runs, may also be important. An alteration in the quantity or quality of these sources may have far reaching effects not only on the organisms that directly consume the allochthonous resource (shredders), but also on other functional feeding groups. Allochthonous resources directly and indirectly change stream microbial communities, which are used by consumers with potential changes to their life histories and behavior traits. The objective of my research was to determine the influence allochthonous resources have on stream -

Ohio EPA Macroinvertebrate Taxonomic Level December 2019 1 Table 1. Current Taxonomic Keys and the Level of Taxonomy Routinely U
Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Table 1. Current taxonomic keys and the level of taxonomy routinely used by the Ohio EPA in streams and rivers for various macroinvertebrate taxonomic classifications. Genera that are reasonably considered to be monotypic in Ohio are also listed. Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Species Pennak 1989, Thorp & Rogers 2016 Porifera If no gemmules are present identify to family (Spongillidae). Genus Thorp & Rogers 2016 Cnidaria monotypic genera: Cordylophora caspia and Craspedacusta sowerbii Platyhelminthes Class (Turbellaria) Thorp & Rogers 2016 Nemertea Phylum (Nemertea) Thorp & Rogers 2016 Phylum (Nematomorpha) Thorp & Rogers 2016 Nematomorpha Paragordius varius monotypic genus Thorp & Rogers 2016 Genus Thorp & Rogers 2016 Ectoprocta monotypic genera: Cristatella mucedo, Hyalinella punctata, Lophopodella carteri, Paludicella articulata, Pectinatella magnifica, Pottsiella erecta Entoprocta Urnatella gracilis monotypic genus Thorp & Rogers 2016 Polychaeta Class (Polychaeta) Thorp & Rogers 2016 Annelida Oligochaeta Subclass (Oligochaeta) Thorp & Rogers 2016 Hirudinida Species Klemm 1982, Klemm et al. 2015 Anostraca Species Thorp & Rogers 2016 Species (Lynceus Laevicaudata Thorp & Rogers 2016 brachyurus) Spinicaudata Genus Thorp & Rogers 2016 Williams 1972, Thorp & Rogers Isopoda Genus 2016 Holsinger 1972, Thorp & Rogers Amphipoda Genus 2016 Gammaridae: Gammarus Species Holsinger 1972 Crustacea monotypic genera: Apocorophium lacustre, Echinogammarus ischnus, Synurella dentata Species (Taphromysis Mysida Thorp & Rogers 2016 louisianae) Crocker & Barr 1968; Jezerinac 1993, 1995; Jezerinac & Thoma 1984; Taylor 2000; Thoma et al. Cambaridae Species 2005; Thoma & Stocker 2009; Crandall & De Grave 2017; Glon et al. 2018 Species (Palaemon Pennak 1989, Palaemonidae kadiakensis) Thorp & Rogers 2016 1 Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Informal grouping of the Arachnida Hydrachnidia Smith 2001 water mites Genus Morse et al. -

Dna Barcodes, Partitioned Phylogenetic Models, And
LARGE DATASETS AND TRICHOPTERA PHYLOGENETICS: DNA BARCODES, PARTITIONED PHYLOGENETIC MODELS, AND THE EVOLUTION OF PHRYGANEIDAE By PAUL BRYAN FRANDSEN A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Karl M. Kjer And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey OCTOBER 2015 ABSTRACT OF THE DISSERTATION Large datasets and Trichoptera phylogenetics: DNA barcodes, partitioned phylogenetic models, and the evolution of Phryganeidae By PAUL BRYAN FRANDSEN Dissertation Director: Karl M. Kjer Large datasets in phylogenetics—those with a large number of taxa, e.g. DNA barcode data sets, and those with a large amount of sequence data per taxon, e.g. data sets generated from high throughput sequencing—pose both exciting possibilities and interesting analytical problems. The analysis of both types of large datasets is explored in this dissertation. First, the use of DNA barcodes in phylogenetics is investigated via the generation of phylogenetic trees for known monophyletic clades. Barcodes are found to be useful in shallow scale phylogenetic analyses when given a well-supported scaffold on which to place them. One of the analytical challenges posed by large phylogenetic datasets is the selection of appropriate partitioned models of molecular evolution. The most commonly used model partitioning strategies can fail to characterize the true variation of the evolutionary process and this effect can be exacerbated when applied to large datasets. A new, scalable algorithm for the automatic selection ! ii! of partitioned models of molecular evolution is proposed with an eye toward reducing systematic error in phylogenomics. -
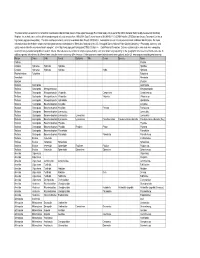
This Table Contains a Taxonomic List of Benthic Invertebrates Collected from Streams in the Upper Mississippi River Basin Study
This table contains a taxonomic list of benthic invertebrates collected from streams in the Upper Mississippi River Basin study unit as part of the USGS National Water Quality Assessemnt (NAWQA) Program. Invertebrates were collected from woody snags in selected streams from 1996-2004. Data Retreival occurred 26-JAN-06 11.10.25 AM from the USGS data warehouse (Taxonomic List Invert http://water.usgs.gov/nawqa/data). The data warehouse currently contains invertebrate data through 09/30/2002. Invertebrate taxa can include provisional and conditional identifications. For more information about invertebrate sample processing and taxonomic standards see, "Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory -- Processing, taxonomy, and quality control of benthic macroinvertebrate samples", at << http://nwql.usgs.gov/Public/pubs/OFR00-212.html >>. Data Retrieval Precaution: Extreme caution must be exercised when comparing taxonomic lists generated using different search criteria. This is because the number of samples represented by each taxa list will vary depending on the geographic criteria selected for the retrievals. In addition, species lists retrieved at different times using the same criteria may differ because: (1) the taxonomic nomenclature (names) were updated, and/or (2) new samples containing new taxa may Phylum Class Order Family Subfamily Tribe Genus Species Taxon Porifera Porifera Cnidaria Hydrozoa Hydroida Hydridae Hydridae Cnidaria Hydrozoa Hydroida Hydridae Hydra Hydra sp. Platyhelminthes Turbellaria Turbellaria Nematoda Nematoda Bryozoa Bryozoa Mollusca Gastropoda Gastropoda Mollusca Gastropoda Mesogastropoda Mesogastropoda Mollusca Gastropoda Mesogastropoda Viviparidae Campeloma Campeloma sp. Mollusca Gastropoda Mesogastropoda Viviparidae Viviparus Viviparus sp. Mollusca Gastropoda Mesogastropoda Hydrobiidae Hydrobiidae Mollusca Gastropoda Basommatophora Ancylidae Ancylidae Mollusca Gastropoda Basommatophora Ancylidae Ferrissia Ferrissia sp. -

100 Characters
40 Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation: Final Report Leon C. Hinz Jr. and James N. Zahniser Illinois Natural History Survey Prairie Research Institute University of Illinois 30 April 2015 INHS Technical Report 2015 (31) Prepared for: Illinois Department of Natural Resources State Wildlife Grant Program (Project Number T-88-R-001) Unrestricted: for immediate online release. Prairie Research Institute, University of Illinois at Urbana Champaign Brian D. Anderson, Interim Executive Director Illinois Natural History Survey Geoffrey A. Levin, Acting Director 1816 South Oak Street Champaign, IL 61820 217-333-6830 Final Report Project Title: Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation. Project Number: T-88-R-001 Contractor information: University of Illinois at Urbana/Champaign Institute of Natural Resource Sustainability Illinois Natural History Survey 1816 South Oak Street Champaign, IL 61820 Project Period: 1 October 2013—31 September 2014 Principle Investigator: Leon C. Hinz Jr., Ph.D. Stream Ecologist Illinois Natural History Survey One Natural Resources Way, Springfield, IL 62702-1271 217-785-8297 [email protected] Prepared by: Leon C. Hinz Jr. & James N. Zahniser Goals/ Objectives: (1) Review all SGNC listing criteria for currently listed non-mollusk invertebrate species using criteria in Illinois Wildlife Action Plan, (2) Assess current status of species populations, (3) Review criteria for additional species for potential listing as SGNC, (4) Assess stressors to species previously reviewed, (5) Complete draft updates and revisions of IWAP Appendix I and Appendix II for non-mollusk invertebrates. T-88 Final Report Project Title: Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation. -

Appendix Page
NORTH CAROLINA DEPARTMENT OF ENVIRONMENT AND NATURAL RESOURCES Division of Water Quality Environmental Sciences Section August 2004 This page was intentionally left blank NCDENR, Division of Water Quality Basinwide Assessment Report – Cape Fear River Basin - August 2004 1 TABLE OF CONTENTS Page LIST OF APPENDICIES ........................................................................................................................ 5 LIST OF TABLES................................................................................................................................... 7 LIST OF FIGURES .............................................................................................................................. 11 OVERVIEW OF THE WATER QUALITY OF THE CAPE FEAR RIVER BASIN.....................................17 EXECUTIVE SUMMARIES BY PROGRAM AREA.................................................................................27 FISHERIES ...................................................................................................................................... 27 BENTHIC MACROINVERTEBRATES............................................................................................. 30 LAKE ASSESSMENT....................................................................................................................... 32 PHYTOPLANKTON MONITORING................................................................................................. 33 AMBIENT MONITORING................................................................................................................ -

Dosdall and Lehmkuhl 1979 Qev15n1 3 116 CC Released.Pdf
This work is licensed under the Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. STONEFLIES (PLECOPTERA) OF SASKATCHEWAN LLOYD M. DOSDALL1 Biology Department University of Saskatchewan Saskatoon, Saskatchewan S7N0W0 D.M. LEHMKUHL Biology Department University of Saskatchewan Saskatoon, Saskatchewan Quaestiones Entomologicae S7N0W0 15:3-116 1979 Forty-one species, twenty-nine genera and eight families of Plecoptera are recorded from Saskatche wan. Distinguishing characters are given and keys are provided. Species recorded are: Pteronarcys dorsata (Say), Pteronarcella badia (Hagen), Taeniopteryx nivalis (Fitch), Oemopteryx fosketti (Packer), Capnia coloradensis Claassen, Capnia confusa Claassen, Capnia gracilaria Claassen, Capnia vernalis Newport, Paracapnia angulata Hanson, Isocapnia crinita (Needham and Claassen), Isocapnia missourii Ricker, Utacapnia trava (Nebeker and Gaufin), Nemoura rickeri Jewett, Shipsa rotunda (Claassen), Amphinemura linda (Ricker), Zapada cinctipes (Banks), Malenka californica (Claassenj, Podmosta delicatula (Claassen, Paraleuctra vershina Gaufin and Ricker, Leuctra ferruginea (Walker), Acroneuria abnormis (Newman), Acroneuria lycorias (Newman), Hesperoperla pacifica (Banks), C'laassenia sabulosa (Banks), Paragnetina media (Walker), Perlesta placida (Hagen), Isoperla bilineata (Say),