Preimplantation Genetic Testing: Where We Are Today
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Background Document on Preimplantation and Prenatal Genetic Testing
DH-BIO/INF (2015) 6 Background document on preimplantation and prenatal genetic testing Clinical Situation Legal situation1 1 Part II of this document presents the legal situation in Council of Europe member states and will be regularly updated (last update 7 May 2015). The updated text is highlighted in green. TABLE OF CONTENTS Introduction ............................................................................................................... 3 Part I. Preimplantation (PGD) and Prenatal (PND) Genetic Diagnosis. Clinical practice, Trends and Technological Developments ................................................... 4 1. Genetic diseases .............................................................................................. 4 1.1 Monogenic diseases ......................................................................................... 4 1.2 Polygenic diseases or multifactorial diseases ................................................... 5 1.3 Chromosomal diseases .................................................................................... 5 2. Preimplantation genetic diagnosis on embryonic cells ...................................... 5 2.1 General description of the procedure ................................................................ 5 2.2 PGD uses ......................................................................................................... 6 2.2.1 Main uses of PGD for medical indications ......................................................... 6 2.2.2 Use of PGD for the benefit of the health -

Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due To
Original Study Oocyte Cryopreservation for Fertility Preservation in Postpubertal Female Children at Risk for Premature Ovarian Failure Due to Accelerated Follicle Loss in Turner Syndrome or Cancer Treatments K. Oktay MD 1,2,*, G. Bedoschi MD 1,2 1 Innovation Institute for Fertility Preservation and IVF, New York, NY 2 Laboratory of Molecular Reproduction and Fertility Preservation, Obstetrics and Gynecology, New York Medical College, Valhalla, NY abstract Objective: To preliminarily study the feasibility of oocyte cryopreservation in postpubertal girls aged between 13 and 15 years who were at risk for premature ovarian failure due to the accelerated follicle loss associated with Turner syndrome or cancer treatments. Design: Retrospective cohort and review of literature. Setting: Academic fertility preservation unit. Participants: Three girls diagnosed with Turner syndrome, 1 girl diagnosed with germ-cell tumor. and 1 girl diagnosed with lymphoblastic leukemia. Interventions: Assessment of ovarian reserve, ovarian stimulation, oocyte retrieval, in vitro maturation, and mature oocyte cryopreservation. Main Outcome Measure: Response to ovarian stimulation, number of mature oocytes cryopreserved and complications, if any. Results: Mean anti-mullerian€ hormone, baseline follical stimulating hormone, estradiol, and antral follicle counts were 1.30 Æ 0.39, 6.08 Æ 2.63, 41.39 Æ 24.68, 8.0 Æ 3.2; respectively. In Turner girls the ovarian reserve assessment indicated already diminished ovarian reserve. Ovarian stimulation and oocyte cryopreservation was successfully performed in all female children referred for fertility preser- vation. A range of 4-11 mature oocytes (mean 8.1 Æ 3.4) was cryopreserved without any complications. All girls tolerated the procedure well. -

Educational Objectives Current IVF Practice
2/23/2015 Update from the IVF Lab: Preimplantation Genetic Testing Janet McLaren Bouknight, MD Reproductive Endocrinology and Infertility Department of Obstetrics & Gynecology Educational Objectives • Gain familiarity with the procedures and pregnancy outcomes of current in vitro fertilization practices. • Understand the application of Preimplantation Genetic Diagnosis (PGD) for single‐gene disorders. • Appreciate the role of Preimplantation Genetic Screening (PGS) in maximizing IVF cycle efficiency and encouraging elective single embryo transfer. Current IVF Practice 1 2/23/2015 Current IVF Practice 150,000 cycles 65,000 livebirths Current IVF Practice Current IVF Practice All SART Clinics 2012 <35 35‐37 38‐40 41‐42 >42 Pregnancy Rate 47% 38% 30% 20% 9% Live Birth Rate 41% 31% 22% 12% 4% # embryos transfer 1.9 2.0 2.4 2.9 2.9 % Twins 30% 25% 20% 13% 9% • What are the advanced that have contributed to an increase in success in the IVF Lab? • Improved lab conditions • Extended embryo culture • Improvements in embryo cryopreservation 2 2/23/2015 Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions Current IVF Practice: Improved Lab Conditions 3 2/23/2015 Current IVF Practice: Extended Embryo Culture Day 3: Cleavage Stage Day 5: Blastocyst Stage • 8‐cells • 200‐300 cells • 30% implantation rate • 50% implantation rate Increased live birth rates seen in “good prognosis” patients who undergo a Day 5 transfer Current IVF Practice: Extended Embryo Culture Current IVF Practice: Extended Embryo Culture • Supports blastocyst transfer for “good prognosis” patients • Opportunity to consider single embryo transfer • May be associated with a small increased risk of monozygotic twinning 4 2/23/2015 Current IVF Practice: Embryo Cryopreservation Cycles • Improvements in freezing techniques (vitrification) have increased success of cryopreserved embryos. -
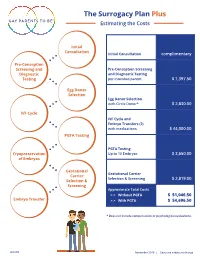
The Surrogacy Plan Plus Estimating the Costs
The Surrogacy Plan Plus Estimating the Costs Initial Consultation Initial Consultation complimentary Pre-Conception Screening and Pre-Conception Screening Diagnostic and Diagnostic Testing Testing per intended parent $ 1,397.50 Egg Donor Selection Egg Donor Selection with Circle Donor* $ 2,830.00 IVF Cycle IVF Cycle and Embryo Transfers (2) with medications $ 44,000.00 PGTA Testing PGTA Testing Cryopreservation Up to 10 Embryos $ 3,650.00 of Embryos Gestational Gestational Carrier Carrier Selection & Screening $ 2,819.00 Selection & Screening Approximate Total Costs > > Without PGTA $ 51,046.50 Embryo Transfer > > With PGTA $ 54,696.50 * Does not include compensation or psychological evaluations. SPP/CD November 2019 | Costs are subject to change The Surrogacy Plan Plus The Surrogacy Plan Plus is available to individuals and couples who want to build a family using a gestational carrrier. In this process, eggs from an egg donor are retrieved and combined with sperm to create embryos. One or two embryos are then transferred into the uterus of the gestational carrier to achieve a pregnancy. How the Surrogacy Plan Plus Works The Surrogacy Plan Plus includes: cycle medication for the oocyte donor and gestational carrier, anesthesia for the retrieval of oocytes, oocyte donor cycle monitoring completed at RMACT, oocyte retrieval, embryology laboratory charges, A simpler approach cryopreservation of embryos, cycle monitoring for the last ultrasound and blood tests to treatment that prior to embryo transfer, embryo transfer into the gestational carrier, and the first year of embryo and specimen storage. If the donor cycle is cancelled prior to retrieval, helps you control the Surrogacy Plan Plus covers the cancellation cost, as well as the cost to rescreen costs with the the same or alternate donor. -

Infertility Services
Medical Policy Assisted Reproductive Services/Infertility Services Document Number: 002 *Commercial and Qualified Health Plans MassHealth Authorization required X No notification or authorization Not covered X *Not all commercial plans cover this service, please check plan’s benefit package to verify coverage. Contents Overview ....................................................................................................................................................... 2 Coverage Guidelines ..................................................................................................................................... 2 MassHealth, and Certain Custom Plans ........................................................................................................ 2 Covered Services/Procedures ....................................................................................................................... 3 General Eligibility Coverage Criteria ............................................................................................................. 3 SERVICE -SPECIFIC INFERTILITY COVERAGE FOR MEMBERS WITH UTERI and OVARIES ............................... 5 Artificial Insemination (AI)/Intrauterine Insemination (IUI) ......................................................................... 5 Conversion from IUI to In Vitro Fertilization (IVF) ........................................................................................ 6 In Vitro Fertilization (IVF) for Infertility ....................................................................................................... -

Cryopreserved Oocyte Versus Fresh Oocyte Assisted Reproductive Technology Cycles, United States, 2013
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Fertil Steril Manuscript Author . Author manuscript; Manuscript Author available in PMC 2018 January 01. Published in final edited form as: Fertil Steril. 2017 January ; 107(1): 110–118. doi:10.1016/j.fertnstert.2016.10.002. Cryopreserved oocyte versus fresh oocyte assisted reproductive technology cycles, United States, 2013 Sara Crawford, Ph.D.a, Sheree L. Boulet, Dr.P.H., M.P.H.a, Jennifer F. Kawwass, M.D.a,b, Denise J. Jamieson, M.D., M.P.H.a, and Dmitry M. Kissin, M.D., M.P.H.a aDivision of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Emory University, Atlanta, Georgia bDivision of Reproductive Endocrinology and Infertility, Department of Gynecology and Obstetrics, Emory University, Atlanta, Georgia Abstract Objective—To compare characteristics, explore predictors, and compare assisted reproductive technology (ART) cycle, transfer, and pregnancy outcomes of autologous and donor cryopreserved oocyte cycles with fresh oocyte cycles. Design—Retrospective cohort study from the National ART Surveillance System. Setting—Fertility treatment centers. Patient(s)—Fresh embryo cycles initiated in 2013 utilizing embryos created with fresh and cryopreserved, autologous and donor oocytes. Intervention(s)—Cryopreservation of oocytes versus fresh. Main Outcomes Measure(s)—Cancellation, implantation, pregnancy, miscarriage, and live birth rates per cycle, transfer, and/or pregnancy. -

Preimplantation Genetic Testing for Aneuploidy (PGT-A): the Biology, the Technology and the Clinical Outcomes
Aust N Z J Obstet Gynaecol 2019; 1–8 DOI: 10.1111/ajo.12960 OPINION Preimplantation genetic testing for aneuploidy (PGT-A): The biology, the technology and the clinical outcomes Hayden Anthony Homer1,2,3 1Christopher Chen Oocyte Biology Research Laboratory, UQ Centre Preimplantation genetic testing for aneuploidy (PGT-A) seeks to identify preim- for Clinical Research, The University plantation embryos with a normal chromosome complement (euploid) during in of Queensland, Brisbane, Queensland, Australia vitro fertilisation (IVF). By sifting out embryos with abnormal chromosome num- 2Reproductive Endocrinology and bers (aneuploid), PGT-A should theoretically improve pregnancy success. Infertility Clinic, Royal Brisbane However, earlier versions of PGT-A were ineffective, and in some cases, detri- & Women's Hospital, Brisbane, Queensland, Australia mental, due to biopsy-induced trauma and because the technology at the time 3Queensland Fertility Group, Brisbane, could analyse only a fraction of all chromosomes. More recently, the emergence Queensland, Australia of technologies enabling all chromosomes to be analysed and a switch to less Correspondence: Professor Hayden traumatic blastocyst-stage biopsy have seen widespread uptake of PGT-A. Anthony Homer, Christopher Chen Assessing the full impact of blastocyst biopsy PGT-A requires consideration of Oocyte Biology Research Laboratory, UQ Centre for Clinical Research, The multiple factors, including embryonic mosaicism, sensitivity of the technological University of Queensland, Building platform used, embryo loss during long-term in vitro culture, embryo cryo- 71/918, Royal Brisbane & Women's Hospital Campus, Herston, QLD 4029, preservation and inter-clinic variability in expertise. Significantly, there hasn‘t Australia. yet been an appropriately designed randomised controlled trial (RCT) of blasto- Email: [email protected] cyst biopsy PGT-A analysed by intention-to- treat that accounts for all these pa- Conflicts of Interest: The author report no conflicts of interest. -

Social Freezing: Pressing Pause on Fertility
International Journal of Environmental Research and Public Health Review Social Freezing: Pressing Pause on Fertility Valentin Nicolae Varlas 1,2 , Roxana Georgiana Bors 1,2, Dragos Albu 1,2, Ovidiu Nicolae Penes 3,*, Bogdana Adriana Nasui 4,* , Claudia Mehedintu 5 and Anca Lucia Pop 6 1 Department of Obstetrics and Gynaecology, Filantropia Clinical Hospital, 011171 Bucharest, Romania; [email protected] (V.N.V.); [email protected] (R.G.B.); [email protected] (D.A.) 2 Department of Obstetrics and Gynaecology, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 3 Department of Intensive Care, University Clinical Hospital, “Carol Davila” University of Medicine and Pharmacy, 37 Dionisie Lupu St., 020021 Bucharest, Romania 4 Department of Community Health, “Iuliu Hat, ieganu” University of Medicine and Pharmacy, 6 Louis Pasteur Street, 400349 Cluj-Napoca, Romania 5 Department of Obstetrics and Gynaecology, Nicolae Malaxa Clinical Hospital, 020346 Bucharest, Romania; [email protected] 6 Department of Clinical Laboratory, Food Safety, “Carol Davila” University of Medicine and Pharmacy, 6 Traian Vuia Street, 020945 Bucharest, Romania; [email protected] * Correspondence: [email protected] (O.N.P.); [email protected] (B.A.N.) Abstract: Increasing numbers of women are undergoing oocyte or tissue cryopreservation for medical or social reasons to increase their chances of having genetic children. Social egg freezing (SEF) allows women to preserve their fertility in anticipation of age-related fertility decline and ineffective fertility treatments at older ages. The purpose of this study was to summarize recent findings focusing on the challenges of elective egg freezing. -

New Advances of Preimplantation and Prenatal Genetic Screening and Noninvasive Testing As a Potential Predictor of Health Status of Babies
Hindawi Publishing Corporation BioMed Research International Volume 2014, Article ID 306505, 8 pages http://dx.doi.org/10.1155/2014/306505 Review Article New Advances of Preimplantation and Prenatal Genetic Screening and Noninvasive Testing as a Potential Predictor of Health Status of Babies Tanya Milachich SAGBAL Dr. Shterev, IVF Unit, Hristo Blagoev 25-31, 1330 Sofia, Bulgaria Correspondence should be addressed to Tanya Milachich; tanya [email protected] Received 24 December 2013; Revised 13 February 2014; Accepted 15 February 2014; Published 24 March 2014 Academic Editor: Irma Virant-Klun Copyright © 2014 Tanya Milachich. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The current morphologically based selection of human embryos for transfer cannot detect chromosome aneuploidies. So far, only biopsy techniques have been able to screen for chromosomal aneuploidies in the in vitro fertilization (IVF) embryos. Preimplantation genetic diagnosis (PGD) or screening (PGS) involves the biopsy of oocyte polar bodies or embryonic cells and has become a routine clinical procedure in many IVF clinics worldwide, including recent development of comprehensive chromosome screening of all 23 pairs of chromosomes by microarrays for aneuploidy screening. The routine preimplantation and prenatal genetic diagnosis (PND) require testing in an aggressive manner. These procedures may be invasive to the growing embryo and fetus and potentially could compromise the clinical outcome. Therefore the aim of this review is to summarize not only the new knowledge on preimplantation and prenatal genetic diagnosis in humans, but also on the development of potential noninvasive embryo and fetal testing that might play an important role in the future. -

Live Births After Polar Body Biopsy and Frozen-Thawed Cleavage Stage Embryo Transfer: Case Report
JBRA Assisted Reproduction 2016;20(4):253-256 doi: 10.5935/1518-0557.20160049 Case Report Live births after polar body biopsy and frozen-thawed cleavage stage embryo transfer: case report Fernando Guimarães1, Matheus Roque1,2, Marcello Valle1, Alessandra Kostolias1, Rodrigo A de Azevedo1, Ciro D Martinhago3, Marcos Sampaio4, Selmo Geber2,4 1ORIGEN – Center for Reproductive Medicine, Rio de Janeiro/RJ - Brazil 2UFMG – Universidade Federal de Minas Gerais, Belo Horizonte/MG - Brazil 3Chromosome Genomic Medicine, São Paulo/SP - Brazil 4ORIGEN – Center for Reproductive Medicine, Belo Horizonte/MG - Brazil ABSTRACT 2002). To perform the biopsy, it is important to cause a Pre-implantation genetic diagnosis (PGD) or screening disruption of the zona pellucida of the oocyte or embryo (PGS) technology, has emerged and developed in the past occurs, which can be performed mechanically, chemically few years, benefiting couples as it allows the selection and or using laser (Brezina et al., 2012). The key point of PGD/ transfer of healthy embryos during IVF treatments. These PGS is to have access to the genetic material to be evalu- techniques can be performed in oocytes (polar-body biop- ated, without compromising the material analyzed and the sy) or embryos (blastomere or trophectoderm biopsy). In quality of the oocyte/embryo (Xu & Montag, 2012). this case report, we describe the first two live births to be Polar body (PB) biopsy was introduced in 1990 (Ver- published in Brazil after a polar-body (PB) biopsy. In case linsky et al., 1990), and it is associated with a less inva- 1, a 42-year-old was submitted to PB biopsy with PGS due sive technique, presenting advantages, because it main- to advanced maternal age and poor ovarian reserve. -

Endometrium and Embryo Implantation: the Hidden Frontier
FINAL PROGRAM AND ABSTRACT 0 1 0 2 , 5 2 - 4 2 r e b m te ep - S Lyon, France Endometrium and Embryo Implantation: the Hidden Frontier Your Continuing Medical Education Partner www.seronosymposia.org Endometrium and Embryo Implantation: the Hidden Frontier GENERAL INFORMATION VENUE Hilton Lyon 70 Quai Charles De Gaulle 69463 Lyon, France Tel: +33-4-7817-5050 LANGUAGE The official language of this Conference will be English. LOCATION Lyon, city of pairs with its 2 rivers, the Rhône and Saône, and its twin hilly districts, Fourvière “the hill of prayers” and Croix Rousse “the hill of workers”. A city proud of its monuments that cohabit marvelously with very contempor ary buildings. Intimate and open, mysterious and influential, impetuous and nonchalant, the home of the famous bouchon bistros, a capital of gastronomy, surrounded by vineyards known the world over, listed by UNESCO as a World Heritage site, a city famous for the invention of the silk and the birthplace of the inventors of cinema. Serono Symposia International Foundation, Annual Conference on: Endometrium and Embryo Implantation: the Hidden Frontier Lyon, France - September 24-25, 2010 AIM OF THE CONFERENCE Implantation is a complex process which requires the orchestration of a series of events involving both the embryo and the endometrium. Due to the complexity of the mechanisms involved and relative lack of information on some of them, it has been defined “black box” of assisted reproduction. Even with the transfer of high quality embryos, implantation rates still remain relatively low, thus failure of implantation is a major factor which influences negatively ART outcomes. -

Triggering Final Oocyte Maturation with Gonadotropin-Releasing
J Assist Reprod Genet (2014) 31:927–932 DOI 10.1007/s10815-014-0248-6 FERTILITY PRESERVATION Triggering final oocyte maturation with gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation: an extended experience Jhansi Reddy & Volkan Turan & Giuliano Bedoschi & Fred Moy & Kutluk Oktay Received: 5 February 2014 /Accepted: 5 May 2014 /Published online: 23 May 2014 # Springer Science+Business Media New York 2014 ABSTRACT Conclusions GnRHa trigger improved cycle outcomes as evi- Purpose To analyze the cycle outcomes and the incidence of denced by the number of mature oocytes and cryopreserved ovarian hyperstimulation syndrome (OHSS), when oocyte embryos, while significantly reducing the risk of OHSS in maturation was triggered by gonadotropin-releasing hormone breast cancer patients undergoing fertility preservation. agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation. Keywords Fertility preservation . Breast cancer . Trigger . Methods One hundred twenty-nine women aged≤45 years, GnRH agonist . hCG diagnosed with stage≤3 breast cancer, with normal ovarian reserve who desired fertility preservation were included in the retrospective cohort study. Ovarian stimulation was achieved utilizing letrozole and gonadotropins. Oocyte maturation was Introduction triggered with GnRHa or hCG. Baseline AMH levels, number of oocytes, maturation and fertilization rates, number of em- Breast cancer is the most common malignancy diagnosed in bryos, and the incidence of OHSS was recorded. women worldwide. Early detection and improvements in Results The serum AMH levels were similar between GnRHa screening have increased the number of premenopausal women and hCG groups (2.7±1.9 vs.