Anatomy and Physiology of Ocular Motor Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

Extraocular Muscles Orbital Muscles
EXTRAOCULAR MUSCLES ORBITAL MUSCLES INTRA- EXTRA- OCULAR OCULAR CILIARY MUSCLES INVOLUNTARY VOLUNTARY 1.Superior tarsal muscle. 1.Levator Palpebrae Superioris 2.Inferior tarsal muscle 2.Superior rectus 3.Inferior rectus 4.Medial rectus 5.Lateral rectus 6.Superior oblique 7.Inferior oblique LEVATOR PALPEBRAE SUPERIORIOS Origin- Inferior surface of lesser wing of sphenoid. Insertion- Upper lamina (Voluntary) - Anterior surface of superior tarsus & skin of upper eyelid. Middle lamina (Involuntary) - Superior margin of superior tarsus. (Superior Tarsus Muscle / Muller muscle) Lower lamina (Involuntary) - Superior conjunctival fornix Nerve Supply :- Voluntary part – Oculomotor Nerve Involuntary part – Sympathetic ACTION :- Elevation of upper eye lid C/S :- Drooping of upper eyelid. Congenital ptosis due to localized myogenic dysgenesis Complete ptosis - Injury to occulomotor nerve. Partial ptosis - disruption of postganglionic sympathetic fibres from superior cervical sympathetic ganglion. Extra ocular Muscles : Origin Levator palpebrae superioris Superior Oblique Superior Rectus Lateral Rectus Medial Rectus Inferior Oblique Inferior Rectus RECTUS MUSCLES : ORIGIN • Arises from a common tendinous ring knows as ANNULUS OF ZINN • Common ring of connective tissue • Anterior to optic foramen • Forms a muscle cone Clinical Significance Retrobulbar neuritis ○ Origin of SUPERIOR AND MEDIAL RECTUS are closely attached to the dural sheath of the optic nerve, which leads to pain during upward & inward movements of the globe. Thyroid orbitopathy ○ Medial & Inf.rectus thicken. especially near the orbital apex - compression of the optic nerve as it enters the optic canal adjacent to the body of the sphenoid bone. Ophthalmoplegia ○ Proptosis occur due to muscle laxity. Medial Rectus Superior Rectus Origin :- Superior limb of the tendonous ring, and optic nerve sheath. -

Treatment of Congenital Ptosis
13 Review Article Page 1 of 13 Treatment of congenital ptosis Vladimir Kratky1,2^ 1Department of Ophthalmology, Queen’s University, Kingston, Canada; 21st Medical Faculty, Charles University, Prague, Czech Republic Correspondence to: Vladimir Kratky, BSc, MD, FRCSC, DABO. Associate Professor of Ophthalmology, Director of Ophthalmic Plastic and Orbital Surgery, Oculoplastics Fellowship Director, Queen’s University, Kingston, Canada; 1st Medical Faculty, Charles University, Prague, Czech Republic. Email: [email protected]. Abstract: Congenital ptosis is an abnormally low position of the upper eyelid, with respect to the visual axis in the primary gaze. It can be present at birth or manifest itself during the first year of life and can be bilateral or unilateral. Additionally, it may be an isolated finding or part of a constellation of signs of a specific syndrome or systemic associations. Depending on how much it interferes with the visual axis, it may be considered as a functional or a cosmetic condition. In childhood, functional ptosis can lead to deprivation amblyopia and astigmatism and needs to be treated. However, even mild ptosis with normal vision can lead to psychosocial problems and correction is also advised, albeit on a less urgent basis. Although, patching and glasses can be prescribed to treat the amblyopia, the mainstay of management is surgical. There are several types of surgical procedure available depending on the severity and etiology of the droopy eyelid. The first part of this paper will review the different categories of congenital ptosis, including more common associated syndromes. The latter part will briefly cover the different surgical approaches, with emphasis on how to choose the correct condition. -

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -
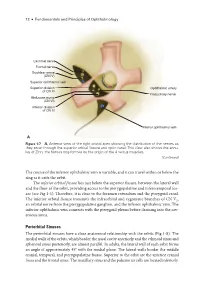
Periorbital Sinuses the Periorbital Sinuses Have a Close Anatomical Relationship with the Orbits (Fig 1-8)
12 ● Fundamentals and Principles of Ophthalmology Lacrimal nerve Frontal nerve Trochlear nerve (CN IV) Superior ophthalmic vein Superior division Ophthalmic artery of CN III Nasociliary nerve Abducens nerve (CN VI) Inferior division of CN III Inferior ophthalmic vein A Figure 1-7 A, Anterior view of the right orbital apex showing the distribution of the nerves as they enter through the superior orbital fissure and optic canal. This view also shows the annu- lus of Zinn, the fibrous ring formed by the origin of the 4 rectus muscles. (Continued) The course of the inferior ophthalmic vein is variable, and it can travel within or below the ring as it exits the orbit. The inferior orbital fissure lies just below the superior fissure, between the lateral wall and the floor of the orbit, providing access to the pterygopalatine and inferotemporal fos- sae (see Fig 1-1). Therefore, it is close to the foramen rotundum and the pterygoid canal. The inferior orbital fissure transmits the infraorbital and zygomatic branches of CN V2, an orbital nerve from the pterygopalatine ganglion, and the inferior ophthalmic vein. The inferior ophthalmic vein connects with the pterygoid plexus before draining into the cav- ernous sinus. Periorbital Sinuses The periorbital sinuses have a close anatomical relationship with the orbits (Fig 1-8). The medial walls of the orbits, which border the nasal cavity anteriorly and the ethmoid sinus and sphenoid sinus posteriorly, are almost parallel. In adults, the lateral wall of each orbit forms an angle of approximately 45° with the medial plane. The lateral walls border the middle cranial, temporal, and pterygopalatine fossae. -

Eye-Movement Studies of Visual Face Perception
Eye-movement studies of visual face perception Joseph Arizpe A dissertation submitted for the degree of Doctor of Philosophy of the University College London Institute of Cognitive Neuroscience University College London 2015 1 Declaration I, Joseph Arizpe, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 Abstract This thesis investigates factors influencing eye-movement patterns during face perception, the relationship of eye-movement patterns to facial recognition performance, and methodological considerations impacting the detection of differences in eye-movement patterns. In particular, in the first study (chapter 2), in which the basis of the other-race effect was investigated, differences in eye- movement patterns during recognition of own- versus other-race (African, Chinese) faces were found for Caucasian participants. However, these eye- movement differences were subtle and analysis-dependent, indicating that the discrepancy in prior reports regarding the presence or absence of such differences are due to variability in statistical sensitivity of analysis methods across studies. The second and third studies (chapters 3 and 4) characterized visuomotor factors, specifically pre-stimulus start position and distance, which strongly influence subsequent eye-movement patterns during face perception. An overall bias in fixation patterns to the opposite side of the face induced by start position and an increasing undershoot of the first ordinal fixation with increasing start distance were found. These visuomotor influences were not specific to faces and did not depend on the predictability of the location of the upcoming stimulus. -

Neuronal Organization in the Inferior Colliculus Revisited with Cell-Type- Dependent Monosynaptic Tracing
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Systems/Circuits Neuronal organization in the inferior colliculus revisited with cell-type- dependent monosynaptic tracing Chenggang Chen1, Mingxiu Cheng1,2, Tetsufumi Ito3 and Sen Song1 1Tsinghua Laboratory of Brain and Intelligence (THBI) and Department of Biomedical Engineering, Beijing Innovation Center for Future Chip, Center for Brain-Inspired Computing Research, McGovern Institute for Brain Research, Tsinghua University, Beijing, 100084, China 2National Institute of Biological Sciences, Beijing, 102206, China 3Anatomy II, School of Medicine, Kanazawa Medical University, Uchinada, Ishikawa, 920-0293, Japan DOI: 10.1523/JNEUROSCI.2173-17.2018 Received: 31 July 2017 Revised: 2 February 2018 Accepted: 7 February 2018 Published: 24 February 2018 Author contributions: C.C., T.I., and S.S. designed research; C.C. and M.C. performed research; C.C. and T.I. analyzed data; C.C. wrote the first draft of the paper; C.C., T.I., and S.S. edited the paper; C.C., T.I., and S.S. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. This work was supported by funding from the National Natural Science Foundation of China (31571095, 91332122, for S.S.), Special Fund of Suzhou-Tsinghua Innovation Leading Action (for S.S.), Beijing Program on the Study of Brain-Inspired Computing System and Related Core Technologies (for S.S.), Beijing Innovation Center for Future Chip (for S.S.), and Chinese Academy of Sciences Institute of Psychology Key Laboratory of Mental Health Open Research Grant (KLMH2012K02, for S.S.), grants from Ministry of Education, Science, and Culture of Japan (KAKENHI grant, Grant numbers 16K07026 and 16H01501; for T.I.), and Takahashi Industrial and Economic Research Foundation (for T.I.). -

Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception
The Journal of Neuroscience, January 28, 2015 • 35(4):1493–1504 • 1493 Behavioral/Cognitive Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception Elena Pretegiani,1,2 Corina Astefanoaei,3 XPierre M. Daye,1,4 Edmond J. FitzGibbon,1 Dorina-Emilia Creanga,3 Alessandra Rufa,2 and XLance M. Optican1 1Laboratory of Sensorimotor Research, NEI, NIH, DHHS, Bethesda, Maryland, 20892-4435, 2EVA-Laboratory, University of Siena, 53100 Siena, Italy, 3Alexandru Ioan Cuza University, Physics Faculty, 700506 Iasi, Romania, and 4Institut du cerveau et de la moelle´pinie e `re (ICM), INSERM UMRS 975, 75013 Paris, France We move our eyes to explore the world, but visual areas determining where to look next (action) are different from those determining what we are seeing (perception). Whether, or how, action and perception are temporally coordinated is not known. The preparation time course of an action (e.g., a saccade) has been widely studied with the gap/overlap paradigm with temporal asynchronies (TA) between peripheral target onset and fixation point offset (gap, synchronous, or overlap). However, whether the subjects perceive the gap or overlap, and when they perceive it, has not been studied. We adapted the gap/overlap paradigm to study the temporal coupling of action and perception. Human subjects made saccades to targets with different TAs with respect to fixation point offset and reported whether they perceived the stimuli as separated by a gap or overlapped in time. Both saccadic and perceptual report reaction times changed in the same way as a function of TA. The TA dependencies of the time change for action and perception were very similar, suggesting a common neural substrate. -

The Complexity and Origins of the Human Eye: a Brief Study on the Anatomy, Physiology, and Origin of the Eye
Running Head: THE COMPLEX HUMAN EYE 1 The Complexity and Origins of the Human Eye: A Brief Study on the Anatomy, Physiology, and Origin of the Eye Evan Sebastian A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2010 THE COMPLEX HUMAN EYE 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ David A. Titcomb, PT, DPT Thesis Chair ______________________________ David DeWitt, Ph.D. Committee Member ______________________________ Garth McGibbon, M.S. Committee Member ______________________________ Marilyn Gadomski, Ph.D. Assistant Honors Director ______________________________ Date THE COMPLEX HUMAN EYE 3 Abstract The human eye has been the cause of much controversy in regards to its complexity and how the human eye came to be. Through following and discussing the anatomical and physiological functions of the eye, a better understanding of the argument of origins can be seen. The anatomy of the human eye and its many functions are clearly seen, through its complexity. When observing the intricacy of vision and all of the different aspects and connections, it does seem that the human eye is a miracle, no matter its origins. Major biological functions and processes occurring in the retina show the intensity of the eye’s intricacy. After viewing the eye and reviewing its anatomical and physiological domain, arguments regarding its origins are more clearly seen and understood. Evolutionary theory, in terms of Darwin’s thoughts, theorized fossilization of animals, computer simulations of eye evolution, and new research on supposed prior genes occurring in lower life forms leading to human life. -

Eye Fields in the Frontal Lobes of Primates
Brain Research Reviews 32Ž. 2000 413±448 www.elsevier.comrlocaterbres Full-length review Eye fields in the frontal lobes of primates Edward J. Tehovnik ), Marc A. Sommer, I-Han Chou, Warren M. Slocum, Peter H. Schiller Department of Brain and CognitiÕe Sciences, Massachusetts Institute of Technology, E25-634, Cambridge, MA 02139, USA Accepted 19 October 1999 Abstract Two eye fields have been identified in the frontal lobes of primates: one is situated dorsomedially within the frontal cortex and will be referred to as the eye field within the dorsomedial frontal cortexŽ. DMFC ; the other resides dorsolaterally within the frontal cortex and is commonly referred to as the frontal eye fieldŽ. FEF . This review documents the similarities and differences between these eye fields. Although the DMFC and FEF are both active during the execution of saccadic and smooth pursuit eye movements, the FEF is more dedicated to these functions. Lesions of DMFC minimally affect the production of most types of saccadic eye movements and have no effect on the execution of smooth pursuit eye movements. In contrast, lesions of the FEF produce deficits in generating saccades to briefly presented targets, in the production of saccades to two or more sequentially presented targets, in the selection of simultaneously presented targets, and in the execution of smooth pursuit eye movements. For the most part, these deficits are prevalent in both monkeys and humans. Single-unit recording experiments have shown that the DMFC contains neurons that mediate both limb and eye movements, whereas the FEF seems to be involved in the execution of eye movements only. -

Bedside Neuro-Otological Examination and Interpretation of Commonly
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.054478 on 24 November 2004. Downloaded from BEDSIDE NEURO-OTOLOGICAL EXAMINATION AND INTERPRETATION iv32 OF COMMONLY USED INVESTIGATIONS RDavies J Neurol Neurosurg Psychiatry 2004;75(Suppl IV):iv32–iv44. doi: 10.1136/jnnp.2004.054478 he assessment of the patient with a neuro-otological problem is not a complex task if approached in a logical manner. It is best addressed by taking a comprehensive history, by a Tphysical examination that is directed towards detecting abnormalities of eye movements and abnormalities of gait, and also towards identifying any associated otological or neurological problems. This examination needs to be mindful of the factors that can compromise the value of the signs elicited, and the range of investigative techniques available. The majority of patients that present with neuro-otological symptoms do not have a space occupying lesion and the over reliance on imaging techniques is likely to miss more common conditions, such as benign paroxysmal positional vertigo (BPPV), or the failure to compensate following an acute unilateral labyrinthine event. The role of the neuro-otologist is to identify the site of the lesion, gather information that may lead to an aetiological diagnosis, and from there, to formulate a management plan. c BACKGROUND Balance is maintained through the integration at the brainstem level of information from the vestibular end organs, and the visual and proprioceptive sensory modalities. This processing takes place in the vestibular nuclei, with modulating influences from higher centres including the cerebellum, the extrapyramidal system, the cerebral cortex, and the contiguous reticular formation (fig 1). -

Glossary of Otologic Terms
Glossary of Otologic Terms. (A) (1) Acoustic reflex ( threshold) (ART). synonym : stapedial reflex ART is the measurement of the change in compliance in the tympanic membrane which occurs when the muscles of the middle ear (tensor tympani, the stapedius muscles) contract when stimulated by a tone at a particular frequency and intensity. Both are striated muscles. Though it is commonly referred to as the stapedial reflex, both the tensor tympani and stapedius muscle are thought to participate in this test. The acoustic reflex threshold (ART) is the sound pressure level (SPL) at which a sound stimulus with a given frequency will trigger the acoustic reflex. The ART is a function of sound pressure level( SPL) and frequency. This acoustic reflex threshold is performed during tympanometry. Persons who have normal hearing have an acoustic reflex threshold (ART) at around 70–100 dB SPL. Persons with conductive hearing loss ( caused by a defect in the middle ear sound transmission mechanism but with an intact tympanic membrane) will likely have an abnormal acoustic reflex threshold depending on the problem in the sound transmission mechanism of the middle ear. The tensor tympani ( nerve supply from the mandibular branch of the trigeminal nerve) pulls the tympanic membrane medially and the stapedius (supplied by a branch of the facial nerve) causes the tympanic membrane to move laterally. Features of the acoustic reflex. · The stapedius muscle contracts bilaterally in normal ears even if only one ear is exposed to sound. · The acoustic reflex protects the inner ear against the harmful effects of low frequency sounds. · When the acoustic reflex is set in motion by sounds of 20 dB above the reflex threshold, the stapedius reflex dampens the intensity of the sound transmitted to the cochlea by about 15 dB.] · Speaking causes the acoustic reflex to take place when a person vocalizes and dampens sound intensities reaching the inner ear by approximately 20 decibels.