En Bloc Removal of the Mandible, the Masticatory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
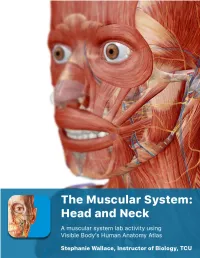
The Muscular System Views
1 PRE-LAB EXERCISES Before coming to lab, get familiar with a few muscle groups we’ll be exploring during lab. Using Visible Body’s Human Anatomy Atlas, go to the Views section. Under Systems, scroll down to the Muscular System views. Select the view Expression and find the following muscles. When you select a muscle, note the book icon in the content box. Selecting this icon allows you to read the muscle’s definition. 1. Occipitofrontalis (epicranius) 2. Orbicularis oculi 3. Orbicularis oris 4. Nasalis 5. Zygomaticus major Return to Muscular System views, select the view Head Rotation and find the following muscles. 1. Sternocleidomastoid 2. Scalene group (anterior, middle, posterior) 2 IN-LAB EXERCISES Use the following modules to guide your exploration of the head and neck region of the muscular system. As you explore the modules, locate the muscles on any charts, models, or specimen available. Please note that these muscles act on the head and neck – those that are located in the neck but act on the back are in a separate section. When reviewing the action of a muscle, it will be helpful to think about where the muscle is located and where the insertion is. Muscle physiology requires that a muscle will “pull” instead of “push” during contraction, and the insertion is the part that will move. Imagine that the muscle is “pulling” on the bone or tissue it is attached to at the insertion. Access 3D views and animated muscle actions in Visible Body’s Human Anatomy Atlas, which will be especially helpful to visualize muscle actions. -

The Role of the Tensor Veli Palatini Muscle in the Development of Cleft Palate-Associated Middle Ear Problems
Clin Oral Invest DOI 10.1007/s00784-016-1828-x REVIEW The role of the tensor veli palatini muscle in the development of cleft palate-associated middle ear problems David S. P. Heidsieck1 & Bram J. A. Smarius1 & Karin P. Q. Oomen2 & Corstiaan C. Breugem1 Received: 8 July 2015 /Accepted: 17 April 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Conclusion More research is warranted to clarify the role of Objective Otitis media with effusion is common in infants the tensor veli palatini muscle in cleft palate-associated with an unrepaired cleft palate. Although its prevalence is Eustachian tube dysfunction and development of middle ear reduced after cleft surgery, many children continue to suffer problems. from middle ear problems during childhood. While the tensor Clinical relevance Optimized surgical management of cleft veli palatini muscle is thought to be involved in middle ear palate could potentially reduce associated middle ear ventilation, evidence about its exact anatomy, function, and problems. role in cleft palate surgery is limited. This study aimed to perform a thorough review of the lit- Keywords Cleft palate . Eustachian tube . Otitis media with erature on (1) the role of the tensor veli palatini muscle in the effusion . Tensor veli palatini muscle Eustachian tube opening and middle ear ventilation, (2) ana- tomical anomalies in cleft palate infants related to middle ear disease, and (3) their implications for surgical techniques used in cleft palate repair. Introduction Materials and methods A literature search on the MEDLINE database was performed using a combination of the keywords Otitis media with effusion is very common in infants with an Btensor veli palatini muscle,^ BEustachian tube,^ Botitis media unrepaired cleft palate under the age of 2 years. -

Questions on Human Anatomy
Standard Medical Text-books. ROBERTS’ PRACTICE OF MEDICINE. The Theory and Practice of Medicine. By Frederick T. Roberts, m.d. Third edi- tion. Octavo. Price, cloth, $6.00; leather, $7.00 Recommended at University of Pennsylvania. Long Island College Hospital, Yale and Harvard Colleges, Bishop’s College, Montreal; Uni- versity of Michigan, and over twenty other medical schools. MEIGS & PEPPER ON CHILDREN. A Practical Treatise on Diseases of Children. By J. Forsyth Meigs, m.d., and William Pepper, m.d. 7th edition. 8vo. Price, cloth, $6.00; leather, $7.00 Recommended at thirty-five of the principal medical colleges in the United States, including Bellevue Hospital, New York, University of Pennsylvania, and Long Island College Hospital. BIDDLE’S MATERIA MEDICA. Materia Medica, for the Use of Students and Physicians. By the late Prof. John B Biddle, m.d., Professor of Materia Medica in Jefferson Medical College, Phila- delphia. The Eighth edition. Octavo. Price, cloth, $4.00 Recommended in colleges in all parts of the UnitedStates. BYFORD ON WOMEN. The Diseases and Accidents Incident to Women. By Wm. H. Byford, m.d., Professor of Obstetrics and Diseases of Women and Children in the Chicago Medical College. Third edition, revised. 164 illus. Price, cloth, $5.00; leather, $6.00 “ Being particularly of use where questions of etiology and general treatment are concerned.”—American Journal of Obstetrics. CAZEAUX’S GREAT WORK ON OBSTETRICS. A practical Text-book on Midwifery. The most complete book now before the profession. Sixth edition, illus. Price, cloth, $6.00 ; leather, $7.00 Recommended at nearly fifty medical schools in the United States. -

CME Anatomy of Aging Face
Published online: 2020-01-15 Free full text on www.ijps.org CME Anatomy of aging face Rakesh Khazanchi, Aditya Aggarwal, Manoj Johar1 Department of Plastic and Cosmetic Surgery, Sir Ganga Ram Hospital, New Delhi - 110 060, 1Fortis Hospital, Noida, UP, India Address for correspondence: Dr. Rakesh Khazanchi, Department of Plastic and Cosmetic Surgery, Sir Ganga Ram Hospital, New Delhi - 110 060, India. E-mail: [email protected] ejuvenation of the face is evolving into a common deposition in regions of body called ‘depots’ procedure in India. This may be attempted by f) Fascial and ligament laxity Reither surgical or non surgical means. Surgical g) Shrinkage of glandular tissue (Salivary glands) rejuvenation of face includes a large variety of procedures h) Skeletal resorption to revert the changes of aging. In the past, face lift operation was done to simply lift the sagging skin rather Facial soft tissues are arranged in concentric layers. than shaping the face. However it often ended up in Skin is the outermost layer and then the basic building giving the patient an ‘operated on’ look producing tight blocks-fat, superficial fascia also known as superficial appearing face. The surgeons have now learnt that aging musculoaponeurotic system (SMAS), deep fascia and the process is a complex process that involves soft tissues as periosteum that covers the facial skeleton. Interspersed well as skeleton of face and is not just sagging of skin. in these layers are vessels, nerves, facial muscles and Therefore in order to get a good result after surgical retaining ligaments. Knowledge of these layers allows facial rejuvenation, it is paramount to understand these the surgeon to dissect in a given anatomic plane without anatomical structures and the effect of aging process on damaging important structures. -

Facial-Stapedial Synkinesis Following Acute Idiopathic Facial Palsy
CASE REPORT Facial-Stapedial Synkinesis Following Acute Idiopathic Facial Palsy Michael Hutz, MD; Margaret Aasen; John Leonetti, MD ABSTRACT complete resolution of their unilateral Introduction: While most patients note a complete resolution of facial paralysis in Bell’s Palsy, facial paralysis, the remaining patients up to 30% will have persistent facial weakness and develop synkinesis. All branches of the manifest persistent paralysis or develop facial nerve are at risk for developing synkinesis, but stapedial synkinesis has rarely been synkinesis, which occurs when a volun- reported in the literature. tary muscle movement causes a simulta- Case Presentation: A 45-year-old man presented with sudden onset, complete right facial neous involuntary contraction of other paralysis. One-and-a-half years later, he had persistent facial weakness and synkinesis. He muscles. The facial nerve is the 7th cra- noted persistent right aural fullness and hearing loss. Audiometry demonstrated facial-stapedial nial nerve and is primarily affected in synkinesis. Bell’s Palsy. It acts to control the muscles Discussion: The patient was diagnosed with stapedial synkinesis based on audiometric find- of facial expression and conveys taste sen- ings by comparing his hearing at rest and with sustained facial mimetic movement. A literature sation to the anterior two-thirds of the review revealed 21 reported cases of this disorder. tongue. Faulty facial nerve regeneration fol- Conclusions: Facial-stapedial synkinesis is an underdiagnosed phenomenon for patients recov- ering from idiopathic facial palsy. Patients who develop facial synkinesis also may have a com- lowing Bell’s Palsy commonly leads to ponent of stapedial synkinesis and should be referred to an otolaryngologist if they complain abnormal muscle contractions of the eye, of any otologic symptoms, such as unilateral hearing loss or tinnitus. -

EDS Awareness in the TMJ Patient
EDS Awareness in the TMJ Patient TMJ and CCI with the EDS Patient “The 50/50” Myofascial Pain Syndrome EDNF, Baltimore, MD August 14,15, 2015 Generation, Diagnosis and Treatment of Head Pain of Musculoskeletal Origin Head pain generated by: • Temporomandibular joint dysfunction • Cervicocranial Instability • Mandibular deviation • Deflection of the Pharyngeal Constrictor Structures Parameters & Observations . The Myofascial Pain Syndrome (MPS) is a description of pain tracking in 200 Ehlers-Danlos patients. Of the 200 patients, 195 were afflicted with this pain referral syndrome pattern. The MPS is in direct association and correlation to Temporomandibular Joint dysfunction and Cervico- Cranial Instability syndromes. Both syndromes are virtually and always correlated. Evaluation of this syndrome was completed after testing was done to rule out complex or life threatening conditions. The Temporomandibular Joint TMJ Dysfunction Symptoms: Deceptively Simple, with Complex Origins 1) Mouth opening, closing with deviation of mandibular condyles. -Menisci that maybe subluxated causing mandibular elevation. -Jaw locking “open” or “closed”. -Inability to “chew”. 2) “Headaches”/”Muscles spasms” (due to decreased vertical height)generated in the temporalis muscle, cheeks areas, under the angle of the jaw. 3) Osseous distortion Pain can be generated in the cheeks, floor of the orbits and/or sinuses due to osseous distortion associated with “bruxism”. TMJ dysfunction cont. (Any of the following motions may produce pain) Pain With: . Limited opening(closed lock): . Less than 33 mm of rotation in either or both joints . Translation- or lack of . Deviations – motion of the mandible to the affected side or none when both joints are affected . Over joint pain with or without motion around or . -

The Myloglossus in a Human Cadaver Study: Common Or Uncommon Anatomical Structure? B
Folia Morphol. Vol. 76, No. 1, pp. 74–81 DOI: 10.5603/FM.a2016.0044 O R I G I N A L A R T I C L E Copyright © 2017 Via Medica ISSN 0015–5659 www.fm.viamedica.pl The myloglossus in a human cadaver study: common or uncommon anatomical structure? B. Buffoli*, M. Ferrari*, F. Belotti, D. Lancini, M.A. Cocchi, M. Labanca, M. Tschabitscher, R. Rezzani, L.F. Rodella Section of Anatomy and Physiopathology, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy [Received: 1 June 2016; Accepted: 18 July 2016] Background: Additional extrinsic muscles of the tongue are reported in literature and one of them is the myloglossus muscle (MGM). Since MGM is nowadays considered as anatomical variant, the aim of this study is to clarify some open questions by evaluating and describing the myloglossal anatomy (including both MGM and its ligamentous counterpart) during human cadaver dissections. Materials and methods: Twenty-one regions (including masticator space, sublin- gual space and adjacent areas) were dissected and the presence and appearance of myloglossus were considered, together with its proximal and distal insertions, vascularisation and innervation. Results: The myloglossus was present in 61.9% of cases with muscular, ligamen- tous or mixed appearance and either bony or muscular insertion. Facial artery pro- vided myloglossal vascularisation in the 84.62% and lingual artery in the 15.38%; innervation was granted by the trigeminal system (buccal nerve and mylohyoid nerve), sometimes (46.15%) with hypoglossal component. Conclusions: These data suggest us to not consider myloglossus as a rare ana- tomical variant. -

MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients After Whiplash Injury
Journal of Clinical Medicine Article MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury Yeon-Hee Lee 1,* , Kyung Mi Lee 2 and Q-Schick Auh 1 1 Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, #613 Hoegi-dong, Dongdaemun-gu, Seoul 02447, Korea; [email protected] 2 Department of Radiology, Kyung Hee University College of Medicine, Kyung Hee University Hospital, #26 Kyunghee-daero, Dongdaemun-gu, Seoul 02447, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-2-958-9409; Fax: +82-2-968-0588 Abstract: Objective: to investigate the change in volume and signal in the masticatory muscles and temporomandibular joint (TMJ) of patients with temporomandibular disorder (TMD) after whiplash injury, based on magnetic resonance imaging (MRI), and to correlate them with other clinical parameters. Methods: ninety patients (64 women, 26 men; mean age: 39.36 ± 15.40 years), including 45 patients with symptoms of TMD after whiplash injury (wTMD), and 45 age- and sex- matched controls with TMD due to idiopathic causes (iTMD) were included. TMD was diagnosed using the study diagnostic criteria for TMD Axis I, and MRI findings of the TMJ and masticatory muscles were investigated. To evaluate the severity of TMD pain and muscle tenderness, we used a visual analog scale (VAS), palpation index (PI), and neck PI. Results: TMD indexes, including VAS, PI, and neck PI were significantly higher in the wTMD group. In the wTMD group, muscle tenderness was highest in the masseter muscle (71.1%), and muscle tenderness in the temporalis (60.0%), lateral pterygoid muscle (LPM) (22.2%), and medial pterygoid muscle (15.6%) was significantly more frequent than that in the iTMD group (all p < 0.05). -
The Mandibular Nerve - Vc Or VIII by Prof
The Mandibular Nerve - Vc or VIII by Prof. Dr. Imran Qureshi The Mandibular nerve is the third and largest division of the trigeminal nerve. It is a mixed nerve. Its sensory root emerges from the posterior region of the semilunar ganglion and is joined by the motor root of the trigeminal nerve. These two nerve bundles leave the cranial cavity through the foramen ovale and unite immediately to form the trunk of the mixed mandibular nerve that passes into the infratemporal fossa. Here, it runs anterior to the middle meningeal artery and is sandwiched between the superior head of the lateral pterygoid and tensor veli palatini muscles. After a short course during which a meningeal branch to the dura mater, and the nerve to part of the medial pterygoid muscle (and the tensor tympani and tensor veli palatini muscles) are given off, the mandibular trunk divides into a smaller anterior and a larger posterior division. The anterior division receives most of the fibres from the motor root and distributes them to the other muscles of mastication i.e. the lateral pterygoid, medial pterygoid, temporalis and masseter muscles. The nerve to masseter and two deep temporal nerves (anterior and posterior) pass laterally above the medial pterygoid. The nerve to the masseter continues outward through the mandibular notch, while the deep temporal nerves turn upward deep to temporalis for its supply. The sensory fibres that it receives are distributed as the buccal nerve. The 1 | P a g e buccal nerve passes between the medial and lateral pterygoids and passes downward and forward to emerge from under cover of the masseter with the buccal artery. -

Head & Neck Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine HEAD‐NECK MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Anterior floor of orbit lateral to Oculomotor nerve (CN III), inferior Abducts, elevates, and laterally Inferior oblique Lateral sclera deep to lateral rectus Ophthalmic artery Extra‐ocular nasolacrimal canal division rotates eyeball Inferior aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Depresses, adducts, and laterally Inferior rectus Common tendinous ring Ophthalmic artery Extra‐ocular corneoscleral junction division rotates eyeball Lateral aspect of eyeball, posterior to Lateral rectus Common tendinous ring Abducent nerve (CN VI) Abducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction Medial aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Medial rectus Common tendinous ring Adducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction division Passes through trochlea, attaches to Body of sphenoid (above optic foramen), Abducts, depresses, and medially Superior oblique superior sclera between superior and Trochlear nerve (CN IV) Ophthalmic artery Extra‐ocular medial to origin of superior rectus rotates eyeball lateral recti Superior aspect of eyeball, posterior to Oculomotor nerve (CN III), superior Elevates, adducts, and medially Superior rectus Common tendinous ring Ophthalmic artery Extra‐ocular the corneoscleral junction division -

New Knowledge Resource for Anatomy Enables Comprehensive Searches of the Literature on the Feeding Muscles of Mammals
RESEARCH ARTICLE Muscle Logic: New Knowledge Resource for Anatomy Enables Comprehensive Searches of the Literature on the Feeding Muscles of Mammals Robert E. Druzinsky1*, James P. Balhoff2, Alfred W. Crompton3, James Done4, Rebecca Z. German5, Melissa A. Haendel6, Anthony Herrel7, Susan W. Herring8, Hilmar Lapp9,10, Paula M. Mabee11, Hans-Michael Muller4, Christopher J. Mungall12, Paul W. Sternberg4,13, a11111 Kimberly Van Auken4, Christopher J. Vinyard5, Susan H. Williams14, Christine E. Wall15 1 Department of Oral Biology, University of Illinois at Chicago, Chicago, Illinois, United States of America, 2 RTI International, Research Triangle Park, North Carolina, United States of America, 3 Organismic and Evolutionary Biology, Harvard University, Cambridge, Massachusetts, United States of America, 4 Division of Biology and Biological Engineering, M/C 156–29, California Institute of Technology, Pasadena, California, United States of America, 5 Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, Ohio, United States of America, 6 Oregon Health and Science University, Portland, Oregon, ’ OPEN ACCESS United States of America, 7 Département d Ecologie et de Gestion de la Biodiversité, Museum National d’Histoire Naturelle, Paris, France, 8 University of Washington, Department of Orthodontics, Seattle, Citation: Druzinsky RE, Balhoff JP, Crompton AW, Washington, United States of America, 9 National Evolutionary Synthesis Center, Durham, North Carolina, Done J, German RZ, Haendel MA, et al. (2016) United States of America, 10 Center for Genomic and Computational Biology, Duke University, Durham, Muscle Logic: New Knowledge Resource for North Carolina, United States of America, 11 Department of Biology, University of South Dakota, Vermillion, South Dakota, United States of America, 12 Genomics Division, Lawrence Berkeley National Laboratory, Anatomy Enables Comprehensive Searches of the Berkeley, California, United States of America, 13 Howard Hughes Medical Institute, M/C 156–29, California Literature on the Feeding Muscles of Mammals. -

Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and Main Principals Of
Yagenich L.V., Kirillova I.I., Siritsa Ye.A. Latin and main principals of anatomical, pharmaceutical and clinical terminology (Student's book) Simferopol, 2017 Contents No. Topics Page 1. UNIT I. Latin language history. Phonetics. Alphabet. Vowels and consonants classification. Diphthongs. Digraphs. Letter combinations. 4-13 Syllable shortness and longitude. Stress rules. 2. UNIT II. Grammatical noun categories, declension characteristics, noun 14-25 dictionary forms, determination of the noun stems, nominative and genitive cases and their significance in terms formation. I-st noun declension. 3. UNIT III. Adjectives and its grammatical categories. Classes of adjectives. Adjective entries in dictionaries. Adjectives of the I-st group. Gender 26-36 endings, stem-determining. 4. UNIT IV. Adjectives of the 2-nd group. Morphological characteristics of two- and multi-word anatomical terms. Syntax of two- and multi-word 37-49 anatomical terms. Nouns of the 2nd declension 5. UNIT V. General characteristic of the nouns of the 3rd declension. Parisyllabic and imparisyllabic nouns. Types of stems of the nouns of the 50-58 3rd declension and their peculiarities. 3rd declension nouns in combination with agreed and non-agreed attributes 6. UNIT VI. Peculiarities of 3rd declension nouns of masculine, feminine and neuter genders. Muscle names referring to their functions. Exceptions to the 59-71 gender rule of 3rd declension nouns for all three genders 7. UNIT VII. 1st, 2nd and 3rd declension nouns in combination with II class adjectives. Present Participle and its declension. Anatomical terms 72-81 consisting of nouns and participles 8. UNIT VIII. Nouns of the 4th and 5th declensions and their combination with 82-89 adjectives 9.