Thermodynamics of a Phase-Driven Proximity Josephson Junction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DFT Quantization of the Gibbs Free Energy of a Quantum Body
DFT quantization of the Gibbs Free Energy of a quantum body S. Selenu (Dated: December 2, 2020) In this article it is introduced a theoretical model made in order to perform calculations of the quantum heat of a body that could be acquired or delivered during a thermal transformation of its quantum states. Here the model is mainly targeted to the electronic structure of matter[1] at the nano and micro scale where DFT models have been frequently developed of the total energy of quantum systems. De fining an Entropy functional S[ρ] makes us able optimizing a free energy G[ρ] of the quantum system at finite temperatures. Due to the generality of the model, the latter can be also applied to several first principles computational codes where ab initio modelling of quantum matter is asked. INTRODUCTION thermal transformations at a fi nite temperature T . This work could then be consid- This article will be focused on a study based on the ered as the begining part of a more general theory can derivation of the quantum electrical heat absorbed or ei- include an ab initio modelling of the first law and second ther released by a physical system made of atoms and law of thermodynamics[13], where heat and free energy electrons either at the nano or at the microscopic scale, variations of a quantum body are taken into account. In extending to it the concept of thermal heat within a DFT fact it is actually considered a quantum system of elec- model. Also, the latter being fundamental in physics, can trons at a given temperature T in thermal equilibrium be employed for a full understanding of the thermal phase with its surrounding. -
Resonance As a Measure of Pairing Correlations in the High-Tc
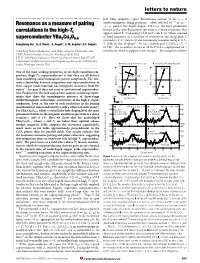
letters to nature ................................................................. lost while magnetic (spin) ¯uctuations centred at the x =0 6±11 Resonance as a measure of pairing antiferromagnetic Bragg positionsÐoften referred to as Q0 = (p, p)±persist. For highly doped (123)O6+x, the most prominent feature in the spin ¯uctuation spectrum is a sharp resonance that correlations in the high-Tc appears below Tc at an energy of 41 meV (refs 6±8). When scanned superconductor YBa Cu O at ®xed frequency as a function of wavevector, the sharp peak is 2 3 6.6 centred at (p, p) (refs 6±8) and its intensity is unaffected by a 11.5- 19 Pengcheng Dai*, H. A. Mook*, G. Aeppli², S. M. Hayden³ & F. DogÏan§ T ®eld in the ab-plane . In our underdoped (123)O6.6 (Tc = 62.7 K)9, the resonance occurs at 34 meV and is superposed on a * Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831-6393, USA continuum which is gapped at low energies11. For frequencies below ² NEC Research Institute, Princeton, New Jersey 08540, USA ³ H. H. Wills Physics Laboratory, University of Bristol, Bristol BS8 1TL, UK § Department of Materials Science and Engineering, University of Washington, Seattle, Washington 98195, USA B rotated 20.6° from the c-axis B along the [3H,-H,0] direction 2.0 2.0 .............................................................................................................................................. a d [H,3H,0] One of the most striking properties of the high-transition-tem- 1.5 1.5 perature (high-Tc) superconductors is that they are all derived [H, (5-H)/3, 0] from insulating antiferromagnetic parent compounds. The inti- 1.0 B ∼ i c-axis 1.0 B i ab-plane mate relationship between magnetism and superconductivity in these copper oxide materials has intrigued researchers from the [0,K,0] (r.l.u.) [0,K,0] (r.l.u.) 0.5 0.5 outset1±4, because it does not exist in conventional superconduc- 20.6° tors. -

Equilibrium Thermodynamics
Equilibrium Thermodynamics Instructor: - Clare Yu (e-mail [email protected], phone: 824-6216) - office hours: Wed from 2:30 pm to 3:30 pm in Rowland Hall 210E Textbooks: - primary: Herbert Callen “Thermodynamics and an Introduction to Thermostatistics” - secondary: Frederick Reif “Statistical and Thermal Physics” - secondary: Kittel and Kroemer “Thermal Physics” - secondary: Enrico Fermi “Thermodynamics” Grading: - weekly homework: 25% - discussion problems: 10% - midterm exam: 30% - final exam: 35% Equilibrium Thermodynamics Material Covered: Equilibrium thermodynamics, phase transitions, critical phenomena (~ 10 first chapters of Callen’s textbook) Homework: - Homework assignments posted on course website Exams: - One midterm, 80 minutes, Tuesday, May 8 - Final, 2 hours, Tuesday, June 12, 10:30 am - 12:20 pm - All exams are in this room 210M RH Course website is at http://eiffel.ps.uci.edu/cyu/p115B/class.html The Subject of Thermodynamics Thermodynamics describes average properties of macroscopic matter in equilibrium. - Macroscopic matter: large objects that consist of many atoms and molecules. - Average properties: properties (such as volume, pressure, temperature etc) that do not depend on the detailed positions and velocities of atoms and molecules of macroscopic matter. Such quantities are called thermodynamic coordinates, variables or parameters. - Equilibrium: state of a macroscopic system in which all average properties do not change with time. (System is not driven by external driving force.) Why Study Thermodynamics ? - Thermodynamics predicts that the average macroscopic properties of a system in equilibrium are not independent from each other. Therefore, if we measure a subset of these properties, we can calculate the rest of them using thermodynamic relations. - Thermodynamics not only gives the exact description of the state of equilibrium but also provides an approximate description (to a very high degree of precision!) of relatively slow processes. -

A Simple Method to Estimate Entropy and Free Energy of Atmospheric Gases from Their Action
Article A Simple Method to Estimate Entropy and Free Energy of Atmospheric Gases from Their Action Ivan Kennedy 1,2,*, Harold Geering 2, Michael Rose 3 and Angus Crossan 2 1 Sydney Institute of Agriculture, University of Sydney, NSW 2006, Australia 2 QuickTest Technologies, PO Box 6285 North Ryde, NSW 2113, Australia; [email protected] (H.G.); [email protected] (A.C.) 3 NSW Department of Primary Industries, Wollongbar NSW 2447, Australia; [email protected] * Correspondence: [email protected]; Tel.: + 61-4-0794-9622 Received: 23 March 2019; Accepted: 26 April 2019; Published: 1 May 2019 Abstract: A convenient practical model for accurately estimating the total entropy (ΣSi) of atmospheric gases based on physical action is proposed. This realistic approach is fully consistent with statistical mechanics, but reinterprets its partition functions as measures of translational, rotational, and vibrational action or quantum states, to estimate the entropy. With all kinds of molecular action expressed as logarithmic functions, the total heat required for warming a chemical system from 0 K (ΣSiT) to a given temperature and pressure can be computed, yielding results identical with published experimental third law values of entropy. All thermodynamic properties of gases including entropy, enthalpy, Gibbs energy, and Helmholtz energy are directly estimated using simple algorithms based on simple molecular and physical properties, without resource to tables of standard values; both free energies are measures of quantum field states and of minimal statistical degeneracy, decreasing with temperature and declining density. We propose that this more realistic approach has heuristic value for thermodynamic computation of atmospheric profiles, based on steady state heat flows equilibrating with gravity. -

Phase Coherence and Andreev Reflection in Topological Insulator Devices
PHYSICAL REVIEW X 4, 041022 (2014) Phase Coherence and Andreev Reflection in Topological Insulator Devices A. D. K. Finck,1 C. Kurter,1 Y. S. Hor, 2 and D. J. Van Harlingen1 1Department of Physics and Materials Research Laboratory, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, USA 2Department of Physics, Missouri University of Science and Technology, Rolla, Missouri 65409, USA (Received 3 June 2014; published 4 November 2014) Topological insulators (TIs) have attracted immense interest because they host helical surface states. Protected by time-reversal symmetry, they are robust to nonmagnetic disorder. When superconductivity is induced in these helical states, they are predicted to emulate p-wave pairing symmetry, with Majorana states bound to vortices. Majorana bound states possess non-Abelian exchange statistics that can be probed through interferometry. Here, we take a significant step towards Majorana interferometry by observing pronounced Fabry-Pérot oscillations in a TI sandwiched between a superconducting and a normal lead. For energies below the superconducting gap, we observe a doubling in the frequency of the oscillations, arising from an additional phase from Andreev reflection. When a magnetic field is applied perpendicular to the TI surface, a number of very sharp and gate-tunable conductance peaks appear at or near zero energy, which has consequences for interpreting spectroscopic probes of Majorana fermions. Our results demonstrate that TIs are a promising platform for exploring phase-coherent transport in a solid-state system. DOI: 10.1103/PhysRevX.4.041022 Subject Areas: Condensed Matter Physics, Superconductivity, Topological Insulators I. INTRODUCTION bound states (MBSs) can be realized in a topological insulator (TI) with induced superconductivity [6–8]. -

Novel Hot Air Engine and Its Mathematical Model – Experimental Measurements and Numerical Analysis
POLLACK PERIODICA An International Journal for Engineering and Information Sciences DOI: 10.1556/606.2019.14.1.5 Vol. 14, No. 1, pp. 47–58 (2019) www.akademiai.com NOVEL HOT AIR ENGINE AND ITS MATHEMATICAL MODEL – EXPERIMENTAL MEASUREMENTS AND NUMERICAL ANALYSIS 1 Gyula KRAMER, 2 Gabor SZEPESI *, 3 Zoltán SIMÉNFALVI 1,2,3 Department of Chemical Machinery, Institute of Energy and Chemical Machinery University of Miskolc, Miskolc-Egyetemváros 3515, Hungary e-mail: [email protected], [email protected], [email protected] Received 11 December 2017; accepted 25 June 2018 Abstract: In the relevant literature there are many types of heat engines. One of those is the group of the so called hot air engines. This paper introduces their world, also introduces the new kind of machine that was developed and built at Department of Chemical Machinery, Institute of Energy and Chemical Machinery, University of Miskolc. Emphasizing the novelty of construction and the working principle are explained. Also the mathematical model of this new engine was prepared and compared to the real model of engine. Keywords: Hot, Air, Engine, Mathematical model 1. Introduction There are three types of volumetric heat engines: the internal combustion engines; steam engines; and hot air engines. The first one is well known, because it is on zenith nowadays. The steam machines are also well known, because their time has just passed, even the elder ones could see those in use. But the hot air engines are forgotten. Our aim is to consider that one. The history of hot air engines is 200 years old. -

Basic Thermodynamics-17ME33.Pdf
Module -1 Fundamental Concepts & Definitions & Work and Heat MODULE 1 Fundamental Concepts & Definitions Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems, Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. Thermodynamic state, state point, state diagram, path and process, quasi-static process, cyclic and non-cyclic processes. Thermodynamic equilibrium; definition, mechanical equilibrium; diathermic wall, thermal equilibrium, chemical equilibrium, Zeroth law of thermodynamics, Temperature; concepts, scales, fixed points and measurements. Work and Heat Mechanics, definition of work and its limitations. Thermodynamic definition of work; examples, sign convention. Displacement work; as a part of a system boundary, as a whole of a system boundary, expressions for displacement work in various processes through p-v diagrams. Shaft work; Electrical work. Other types of work. Heat; definition, units and sign convention. 10 Hours 1st Hour Brain storming session on subject topics Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems 2nd Hour Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. 3rd Hour Thermodynamic state, state point, state diagram, path and process, quasi-static -

Proximity to a Critical Point Driven by Electronic Entropy in Uru2si2
www.nature.com/npjquantmats ARTICLE OPEN Proximity to a critical point driven by electronic entropy in URu2Si2 ✉ Neil Harrison 1 , Satya K. Kushwaha1,2, Mun K. Chan 1 and Marcelo Jaime 1 The strongly correlated actinide metal URu2Si2 exhibits a mean field-like second order phase transition at To ≈ 17 K, yet lacks definitive signatures of a broken symmetry. Meanwhile, various experiments have also shown the electronic energy gap to closely resemble that resulting from hybridization between conduction electron and 5f-electron states. We argue here, using thermodynamic measurements, that the above seemingly incompatible observations can be jointly understood by way of proximity to an entropy-driven critical point, in which the latent heat of a valence-type electronic instability is quenched by thermal excitations across a gap, driving the transition second order. Salient features of such a transition include a robust gap spanning highly degenerate features in the electronic density of states, that is weakly (if at all) suppressed by temperature on approaching To, and an elliptical phase boundary in magnetic field and temperature that is Pauli paramagnetically limited at its critical magnetic field. npj Quantum Materials (2021) 6:24 ; https://doi.org/10.1038/s41535-021-00317-6 1234567890():,; INTRODUCTION no absolute requirement for the magnitude of a hybridization gap URu2Si2 remains of immense interest owing to the possibility to vanish at a phase transition, it need not be thermally of it exhibiting a form of order distinct from that observed -

Thermodynamics the Goal of Thermodynamics Is to Understand How Heat Can Be Converted to Work
Thermodynamics The goal of thermodynamics is to understand how heat can be converted to work Main lesson: Not all the heat energy can be converted to mechanical energy This is because heat energy comes with disorder (entropy), and overall disorder cannot decrease Temperature v T Random directions of velocity Higher temperature means higher velocities v Add up energy of all molecules: Internal Energy of gas U Mechanical energy: all atoms move in the same direction 1 Mv2 2 Statistical mechanics For one atom 1 1 1 1 1 1 3 E = mv2 + mv2 + mv2 = kT + kT + kT = kT h i h2 xi h2 yi h2 z i 2 2 2 2 Ideal gas: No Potential energy from attraction between atoms 3 U = NkT h i 2 v T Pressure v Pressure is caused because atoms T bounce off the wall Lx p − x px ∆px =2px 2L ∆t = x vx ∆p 2mv2 mv2 F = x = x = x ∆t 2Lx Lx ∆p 2mv2 mv2 F = x = x = x ∆t 2Lx Lx 1 mv2 =2 kT = kT h xi ⇥ 2 kT F = Lx Pressure v F kT 1 kT A = LyLz P = = = A Lx LyLz V NkT Many particles P = V Lx PV = NkT Volume V = LxLyLz Work Lx ∆V = A ∆Lx v A = LyLz Work done BY the gas ∆W = F ∆Lx We can write this as ∆W =(PA) ∆Lx = P ∆V This is useful because the body could have a generic shape Internal energy of gas decreases U U ∆W ! − Gas expands, work is done BY the gas P dW = P dV Work done is Area under curve V P Gas is pushed in, work is done ON the gas dW = P dV − Work done is negative of Area under curve V By convention, we use POSITIVE sign for work done BY the gas Getting work from Heat Gas expands, work is done BY the gas P dW = P dV V Volume in increases Internal energy decreases .. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -

Statistical Thermodynamics - Fall 2009
1 Statistical Thermodynamics - Fall 2009 Professor Dmitry Garanin Thermodynamics September 9, 2012 I. PREFACE The course of Statistical Thermodynamics consist of two parts: Thermodynamics and Statistical Physics. These both branches of physics deal with systems of a large number of particles (atoms, molecules, etc.) at equilibrium. 3 19 One cm of an ideal gas under normal conditions contains NL =2.69 10 atoms, the so-called Loschmidt number. Although one may describe the motion of the atoms with the help of× Newton’s equations, direct solution of such a large number of differential equations is impossible. On the other hand, one does not need the too detailed information about the motion of the individual particles, the microscopic behavior of the system. One is rather interested in the macroscopic quantities, such as the pressure P . Pressure in gases is due to the bombardment of the walls of the container by the flying atoms of the contained gas. It does not exist if there are only a few gas molecules. Macroscopic quantities such as pressure arise only in systems of a large number of particles. Both thermodynamics and statistical physics study macroscopic quantities and relations between them. Some macroscopics quantities, such as temperature and entropy, are non-mechanical. Equilibruim, or thermodynamic equilibrium, is the state of the system that is achieved after some time after time-dependent forces acting on the system have been switched off. One can say that the system approaches the equilibrium, if undisturbed. Again, thermodynamic equilibrium arises solely in macroscopic systems. There is no thermodynamic equilibrium in a system of a few particles that are moving according to the Newton’s law. -

Szilard-1929.Pdf
In memory of Leo Szilard, who passed away on May 30,1964, we present an English translation of his classical paper ober die Enfropieuerminderung in einem thermodynamischen System bei Eingrifen intelligenter Wesen, which appeared in the Zeitschrift fur Physik, 1929,53,840-856. The publica- tion in this journal of this translation was approved by Dr. Szilard before he died, but he never saw the copy. At Mrs. Szilard’s request, Dr. Carl Eckart revised the translation. This is one of the earliest, if not the earliest paper, in which the relations of physical entropy to information (in the sense of modem mathematical theory of communication) were rigorously demonstrated and in which Max- well’s famous demon was successfully exorcised: a milestone in the integra- tion of physical and cognitive concepts. ON THE DECREASE OF ENTROPY IN A THERMODYNAMIC SYSTEM BY THE INTERVENTION OF INTELLIGENT BEINGS by Leo Szilard Translated by Anatol Rapoport and Mechthilde Knoller from the original article “Uber die Entropiever- minderung in einem thermodynamischen System bei Eingriffen intelligenter Wesen.” Zeitschrift fur Physik, 1929, 65, 840-866. 0*3 resulting quantity of entropy. We find that The objective of the investigation is to it is exactly as great as is necessary for full find the conditions which apparently allow compensation. The actual production of the construction of a perpetual-motion ma- entropy in connection with the measure- chine of the second kind, if one permits an ment, therefore, need not be greater than intelligent being to intervene in a thermo- Equation (1) requires. dynamic system. When such beings make F(J measurements, they make the system behave in a manner distinctly different from the way HERE is an objection, already historical, a mechanical system behaves when left to Tagainst the universal validity of the itself.