Ch 19. the First Law of Thermodynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Hot Air Engine and Its Mathematical Model – Experimental Measurements and Numerical Analysis
POLLACK PERIODICA An International Journal for Engineering and Information Sciences DOI: 10.1556/606.2019.14.1.5 Vol. 14, No. 1, pp. 47–58 (2019) www.akademiai.com NOVEL HOT AIR ENGINE AND ITS MATHEMATICAL MODEL – EXPERIMENTAL MEASUREMENTS AND NUMERICAL ANALYSIS 1 Gyula KRAMER, 2 Gabor SZEPESI *, 3 Zoltán SIMÉNFALVI 1,2,3 Department of Chemical Machinery, Institute of Energy and Chemical Machinery University of Miskolc, Miskolc-Egyetemváros 3515, Hungary e-mail: [email protected], [email protected], [email protected] Received 11 December 2017; accepted 25 June 2018 Abstract: In the relevant literature there are many types of heat engines. One of those is the group of the so called hot air engines. This paper introduces their world, also introduces the new kind of machine that was developed and built at Department of Chemical Machinery, Institute of Energy and Chemical Machinery, University of Miskolc. Emphasizing the novelty of construction and the working principle are explained. Also the mathematical model of this new engine was prepared and compared to the real model of engine. Keywords: Hot, Air, Engine, Mathematical model 1. Introduction There are three types of volumetric heat engines: the internal combustion engines; steam engines; and hot air engines. The first one is well known, because it is on zenith nowadays. The steam machines are also well known, because their time has just passed, even the elder ones could see those in use. But the hot air engines are forgotten. Our aim is to consider that one. The history of hot air engines is 200 years old. -

Muddiest Point – Entropy and Reversible I Am Confused About Entropy and How It Is Different in a Reversible Versus Irreversible Case
1 Muddiest Point { Entropy and Reversible I am confused about entropy and how it is different in a reversible versus irreversible case. Note: Some of the discussion below follows from the previous muddiest points comment on the general idea of a reversible and an irreversible process. You may wish to have a look at that comment before reading this one. Let's talk about entropy first, and then we will consider how \reversible" gets involved. Generally we divide the universe into two parts, a system (what we are studying) and the surrounding (everything else). In the end the total change in the entropy will be the sum of the change in both, dStotal = dSsystem + dSsurrounding: This total change of entropy has only two possibilities: Either there is no spontaneous change (equilibrium) and dStotal = 0, or there is a spontaneous change because we are not at equilibrium, and dStotal > 0. Of course the entropy change of each piece, system or surroundings, can be positive or negative. However, the second law says the sum must be zero or positive. Let's start by thinking about the entropy change in the system and then we will add the entropy change in the surroundings. Entropy change in the system: When you consider the change in entropy for a process you should first consider whether or not you are looking at an isolated system. Start with an isolated system. An isolated system is not able to exchange energy with anything else (the surroundings) via heat or work. Think of surrounding the system with a perfect, rigid insulating blanket. -

Basic Thermodynamics-17ME33.Pdf
Module -1 Fundamental Concepts & Definitions & Work and Heat MODULE 1 Fundamental Concepts & Definitions Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems, Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. Thermodynamic state, state point, state diagram, path and process, quasi-static process, cyclic and non-cyclic processes. Thermodynamic equilibrium; definition, mechanical equilibrium; diathermic wall, thermal equilibrium, chemical equilibrium, Zeroth law of thermodynamics, Temperature; concepts, scales, fixed points and measurements. Work and Heat Mechanics, definition of work and its limitations. Thermodynamic definition of work; examples, sign convention. Displacement work; as a part of a system boundary, as a whole of a system boundary, expressions for displacement work in various processes through p-v diagrams. Shaft work; Electrical work. Other types of work. Heat; definition, units and sign convention. 10 Hours 1st Hour Brain storming session on subject topics Thermodynamics definition and scope, Microscopic and Macroscopic approaches. Some practical applications of engineering thermodynamic Systems 2nd Hour Characteristics of system boundary and control surface, examples. Thermodynamic properties; definition and units, intensive and extensive properties. 3rd Hour Thermodynamic state, state point, state diagram, path and process, quasi-static -

Thermodynamics the Goal of Thermodynamics Is to Understand How Heat Can Be Converted to Work
Thermodynamics The goal of thermodynamics is to understand how heat can be converted to work Main lesson: Not all the heat energy can be converted to mechanical energy This is because heat energy comes with disorder (entropy), and overall disorder cannot decrease Temperature v T Random directions of velocity Higher temperature means higher velocities v Add up energy of all molecules: Internal Energy of gas U Mechanical energy: all atoms move in the same direction 1 Mv2 2 Statistical mechanics For one atom 1 1 1 1 1 1 3 E = mv2 + mv2 + mv2 = kT + kT + kT = kT h i h2 xi h2 yi h2 z i 2 2 2 2 Ideal gas: No Potential energy from attraction between atoms 3 U = NkT h i 2 v T Pressure v Pressure is caused because atoms T bounce off the wall Lx p − x px ∆px =2px 2L ∆t = x vx ∆p 2mv2 mv2 F = x = x = x ∆t 2Lx Lx ∆p 2mv2 mv2 F = x = x = x ∆t 2Lx Lx 1 mv2 =2 kT = kT h xi ⇥ 2 kT F = Lx Pressure v F kT 1 kT A = LyLz P = = = A Lx LyLz V NkT Many particles P = V Lx PV = NkT Volume V = LxLyLz Work Lx ∆V = A ∆Lx v A = LyLz Work done BY the gas ∆W = F ∆Lx We can write this as ∆W =(PA) ∆Lx = P ∆V This is useful because the body could have a generic shape Internal energy of gas decreases U U ∆W ! − Gas expands, work is done BY the gas P dW = P dV Work done is Area under curve V P Gas is pushed in, work is done ON the gas dW = P dV − Work done is negative of Area under curve V By convention, we use POSITIVE sign for work done BY the gas Getting work from Heat Gas expands, work is done BY the gas P dW = P dV V Volume in increases Internal energy decreases .. -

Isothermal Process It Is the Process in Which Other Physical Quantities Might Change but the Temperature of the System Remains Or Is Forced to Remain Constant
Sajit Chandra Shakya Department of Physics Kathmandu Don Bosco College New Baneshwor, Kathmandu Isothermal process It is the process in which other physical quantities might change but the temperature of the system remains or is forced to remain constant. For example, the constant temperature of human body. Under constant temperature, the volume of a gas system is inversely proportional to the pressure applied, the phenomena being called Boyle's Law, written in symbols as 1 V ∝ P 1 Or, V = KB× where KB is a constant quantity. P Or, PV = KB …………….. (i) This means whatever be the values of volume and pressure, their product will be constant. So, P1V1 = KB, P2V2 = KB, P3V3 = KB, etc, Or, P1V1 = P2V2 = P3V3, etc. The requirements for an isothermal process are as follows: 1. The process should be carried very slowly so that there is an ample time for compensation of heat in case of any loss or addition. 2. The boundaries of the system should be highly conducting so that there is a path for heat to flow into or flow away from a closed space in case of any energy loss or oversupply. 3. The boundaries should be made very thin because the resistance of the substance for heat conduction will be less for thin boundaries. Since in an isothermal change, the temperature remains constant, the internal energy also does not change, i.e. dU = 0. So if dQ amount of heat is given to a system which undergoes isothermal change, the relation for the first law of thermodynamics would be dQ = dU + dW Or, dQ = 0 + PdV Or, dQ = PdV This means all the heat supplied will be utilized for performing external work and consequently its value will be very high compared to other processes. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -

The First Law of Thermodynamics Continued Pre-Reading: §19.5 Where We Are
Lecture 7 The first law of thermodynamics continued Pre-reading: §19.5 Where we are The pressure p, volume V, and temperature T are related by an equation of state. For an ideal gas, pV = nRT = NkT For an ideal gas, the temperature T is is a direct measure of the average kinetic energy of its 3 3 molecules: KE = nRT = NkT tr 2 2 2 3kT 3RT and vrms = (v )av = = r m r M p Where we are We define the internal energy of a system: UKEPE=+∑∑ interaction Random chaotic between atoms motion & molecules For an ideal gas, f UNkT= 2 i.e. the internal energy depends only on its temperature Where we are By considering adding heat to a fixed volume of an ideal gas, we showed f f Q = Nk∆T = nR∆T 2 2 and so, from the definition of heat capacity Q = nC∆T f we have that C = R for any ideal gas. V 2 Change in internal energy: ∆U = nCV ∆T Heat capacity of an ideal gas Now consider adding heat to an ideal gas at constant pressure. By definition, Q = nCp∆T and W = p∆V = nR∆T So from ∆U = Q W − we get nCV ∆T = nCp∆T nR∆T − or Cp = CV + R It takes greater heat input to raise the temperature of a gas a given amount at constant pressure than constant volume YF §19.4 Ratio of heat capacities Look at the ratio of these heat capacities: we have f C = R V 2 and f + 2 C = C + R = R p V 2 so C p γ = > 1 CV 3 For a monatomic gas, CV = R 3 5 2 so Cp = R + R = R 2 2 C 5 R 5 and γ = p = 2 = =1.67 C 3 R 3 YF §19.4 V 2 Problem An ideal gas is enclosed in a cylinder which has a movable piston. -

Supercritical CO 2 Cycles for Gas Turbine
Power Gen International December 8-10, Las Vegas, Nevada SUPERCRITICAL CO2 CYCLES FOR GAS TURBINE COMBINED CYCLE POWER PLANTS Timothy J. Held Chief Technology Officer Echogen Power Systems, LLC Akron, Ohio [email protected] ABSTRACT Supercritical carbon dioxide (sCO2) used as the working fluid in closed loop power conversion cycles offers significant advantages over steam and organic fluid based Rankine cycles. Echogen Power Systems LLC has developed several variants of sCO2 cycles that are optimized for bottoming and heat recovery applications. In contrast to cycles used in previous nuclear and CSP studies, these cycles are highly effective in extracting heat from a sensible thermal source such as gas turbine exhaust or industrial process waste heat, and then converting it to power. In this study, conceptual designs of sCO2 heat recovery systems are developed for gas turbine combined cycle (GTCC) power generation over a broad range of system sizes, ranging from distributed generation (~5MW) to utility scale (> 500MW). Advanced cycle simulation tools employing non-linear multivariate constrained optimization processes are combined with system and plant cost models to generate families of designs with different cycle topologies. The recently introduced EPS100 [1], the first commercial-scale sCO2 heat recovery engine, is used to validate the results of the cost and performance models. The results of the simulation process are shown as system installed cost as a function of power, which allows objective comparisons between different cycle architectures, and to other power generation technologies. Comparable system cost and performance studies for conventional steam-based GTCC are presented on the basis of GT-Pro™ simulations [2]. -

Removing the Mystery of Entropy and Thermodynamics – Part II Harvey S
Table IV. Example of audit of energy loss in collision (with a mass attached to the end of the spring). Removing the Mystery of Entropy and Thermodynamics – Part II Harvey S. Leff, Reed College, Portland, ORa,b Part II of this five-part series is focused on further clarification of entropy and thermodynamics. We emphasize that entropy is a state function with a numerical value for any substance in thermodynamic equilibrium with its surroundings. The inter- pretation of entropy as a “spreading function” is suggested by the Clausius algorithm. The Mayer-Joule principle is shown to be helpful in understanding entropy changes for pure work processes. Furthermore, the entropy change when a gas expands or is compressed, and when two gases are mixed, can be understood qualitatively in terms of spatial energy spreading. The question- answer format of Part I1 is continued, enumerating main results in Key Points 2.1-2.6. • What is the significance of entropy being a state Although the linear correlation in Fig. 1 is quite striking, the function? two points to be emphasized here are: (i) the entropy value In Part I, we showed that the entropy of a room temperature of each solid is dependent on the energy added to and stored solid can be calculated at standard temperature and pressure by it, and (ii) the amount of energy needed for the heating using heat capacity data from near absolute zero, denoted by T process from T = 0 + K to T = 298.15 K differs from solid to = 0+, to room temperature, Tf = 298.15 K. -
Thermodynamic Propertiesproperties
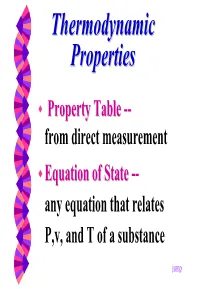
ThermodynamicThermodynamic PropertiesProperties PropertyProperty TableTable -- from direct measurement EquationEquation ofof StateState -- any equation that relates P,v, and T of a substance jump ExerciseExercise 33--1212 A bucket containing 2 liters of R-12 is left outside in the atmosphere (0.1 MPa) a) What is the R-12 temperature assuming it is in the saturated state. b) the surrounding transfer heat at the rate of 1KW to the liquid. How long will take for all R-12 vaporize? See R-12 (diclorindifluormethane) on Table A-2 SolutionSolution -- pagepage 11 Part a) From table A-2, at the saturation pressure of 0.1 MPa one finds: • Tsaturation = - 30oC 3 • vliq = 0.000672 m /kg 3 • vvap = 0.159375 m /kg • hlv = 165KJ/kg (vaporization heat) SolutionSolution -- pagepage 22 Part b) The mass of R-12 is m = Volume/vL, m = 0.002/0.000672 = 2.98 kg The vaporization energy: Evap = vap energy * mass = 165*2.98 = 492 KJ Time = Heat/Power = 492 sec or 8.2 min GASGAS PROPERTIESPROPERTIES Ideal -Gas Equation of State M PV = nR T; n = u mol Universal gas constant is given on Ru = 8.31434 kJ/kmol-K = 8.31434 kPa-m3/kmol-k = 0.0831434 bar-m3/kmol-K = 82.05 L-atm/kmol-K = 1.9858 Btu/lbmol-R = 1545.35 ft-lbf/lbmol-R = 10.73 psia-ft3/lbmol-R EExamplexample Determine the particular gas constant for air (28.97 kg/kmol) and hydrogen (2.016 kg/kmol). kJ 8.1417 kJ = Ru = kmol − K = 0.287 Rair kg M 28.97 kg − K kmol kJ 8.1417 kJ = kmol − K = 4.124 Rhydrogen kg 2.016 kg − K kmol IdealIdeal GasGas “Law”“Law” isis aa simplesimple EquationEquation ofof StateState PV = MRT Pv = RT PV = NRuT P V P V 1 1 = 2 2 T1 T2 QuestionQuestion …...….. -

“Fabrication of Stirling Engine”
“Fabrication of Stirling Engine” Sibabrata Mohanty*,Debasis Pattnayak** , Ashik Dash*** ,Raju Singh**** , Chandrakant Bhardwaj***** , Sk Firdosh Akhtar****** *Assistant Professor, Department of Mechanical Engineering, Gandhi Institute of Engineering and Technology, Gunupur, Rayagada Odisha, **,***,****,*****,&****** B.Tech Mechanical Students, Gandhi Institute of Engineering and Technology, Gunupur, Rayagada Odisha ABSTRACT The performance of Stirling engines meets the demands of the efficient use of energy and environmental security and therefore they are the subject of much current interest. Hence, the development and investigation of Stirling engine have come to the attention of many scientific institutes and commercial companies. The Stirling engine is both practically and theoretically a significant device, its practical virtue is simple, reliable and safe which was recognized for a full century following its invention by Robert Stirling in 1816. The engine operates on a closed thermodynamic cycle, which is reversible. The objective of this project paper is to provide fundamental information and present a detailed review of the efforts taken by us for the development of the Stirling cycle engine and techniques used for engine analysis. A number of attempts have been made by us to build and improve the performance of Stirling engines. It is seen that for successful operation of engine system with good efficiency a careful design, proper selection of drive mechanism and engine configuration is essential. This project paper indicates that a Stirling cycle engine working with relatively low temperature with air or helium as working fluid is potentially attractive engines of the future, especially solar-powered low-temperature differential Stirling engines. Keywords: Stirling engines, Stirling cycle, thermodynamic cycle 1. -

12/8 and 12/10/2010
PY105 C1 1. Help for Final exam has been posted on WebAssign. 2. The Final exam will be on Wednesday December 15th from 6-8 pm. First Law of Thermodynamics 3. You will take the exam in multiple rooms, divided as follows: SCI 107: Abbasi to Fasullo, as well as Khajah PHO 203: Flynn to Okuda, except for Khajah SCI B58: Ordonez to Zhang 1 2 Heat and Work done by a Gas Thermodynamics Initial: Consider a cylinder of ideal Thermodynamics is the study of systems involving gas at room temperature. Suppose the piston on top of energy in the form of heat and work. the cylinder is free to move vertically without friction. When the cylinder is placed in a container of hot water, heat Equilibrium: is transferred into the cylinder. Where does the heat energy go? Why does the volume increase? 3 4 The First Law of Thermodynamics The First Law of Thermodynamics The First Law is often written as: Some of the heat energy goes into raising the temperature of the gas (which is equivalent to raising the internal energy of the gas). The rest of it does work by raising the piston. ΔEQWint =− Conservation of energy leads to: QEW=Δ + int (the first law of thermodynamics) This form of the First Law says that the change in internal energy of a system is Q is the heat added to a system (or removed if it is negative) equal to the heat supplied to the system Eint is the internal energy of the system (the energy minus the work done by the system (usually associated with the motion of the atoms and/or molecules).