A Functional Spiking Neuronal Network for Tactile Sensing Pathway to Process Edge Orientation Adel Parvizi‑Fard1, Mahmood Amiri2*, Deepesh Kumar3, Mark M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MITOCW | 9: Receptive Fields - Intro to Neural Computation
MITOCW | 9: Receptive Fields - Intro to Neural Computation MICHALE FEE: So today, we're going to introduce a new topic, which is related to the idea of fine- tuning curves, and that is the notion of receptive fields. So most of you have probably been, at least those of you who've taken 9.01 or 9.00 maybe, have been exposed to the idea of what a receptive field is. The idea is basically that in sensory systems neurons receive input from the sensory periphery, and neurons generally have some kind of sensory stimulus that causes them to spike. And so one of the classic examples of how to find receptive fields comes from the work of Huble and Wiesel. So I'll show you some movies made from early experiments of Huble-Wiesel where they are recording in the visual cortex of the cat. So they place a fine metal electrode into a primary visual cortex, and they present. So then they anesthetize the cat so the cat can't move. They open the eye, and the cat's now looking at a screen that looks like this, where they play a visual stimulus. And they actually did this with essentially a slide projector that they could put a card in front of that had a little hole in it, for example, that allowed a spot of light to project onto the screen. And then they can move that spot of light around while they record from neurons in visual cortex and present different visual stimuli to the retina. -

On the Existence of Mechanoreceptors Within the Neurovascular Unit of the Rodent and Rabbit Brain
bioRxiv preprint doi: https://doi.org/10.1101/480921; this version posted November 28, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. On the existence of mechanoreceptors within the neurovascular unit of the rodent and rabbit brain Jorge Larriva-Sahd, Martha León-Olea (*), Víctor Vargas-Barroso (**), Alfredo Varela-Echavarría and Luis Concha Departments of Developmental Biology and Physiology, Instituto de Neurobiología. Campus Juriquilla. Universidad Nacional Autónoma de México. Boulevard Universitario 3001, Juriquilla, Querétaro, CP 76230, México. (*) Department of Functional Neuromorphology, Instituto Mexicano de Psiquiatría “Ramón de la Fuente Muñiz” Av México Xochimilco 101, Delegación Tlalpan CP14370, México DF, México. (**) Present address: Institute of Science and Technology Austria (IST), Am Campus 1, 3400, Klosterneuburg, Austria.Correspondence: Correspondence: Jorge Larriva-Sahd e-mail: [email protected] Telephone: 5256-234030 Orcid ID: 0000-0002-7254-0773 bioRxiv preprint doi: https://doi.org/10.1101/480921; this version posted November 28, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Abstract. We describe a set of perivascular interneurons (PINs) originating a series of fibro-vesicular complexes (FVCs) throughout the gray matter of the adult rabbit and rat brain. PINs-FVCs are ubiquitous throughout the brain vasculature as defined in Golgi-impregnated specimens. -

Chemoreception
Senses 5 SENSES live version • discussion • edit lesson • comment • report an error enses are the physiological methods of perception. The senses and their operation, classification, Sand theory are overlapping topics studied by a variety of fields. Sense is a faculty by which outside stimuli are perceived. We experience reality through our senses. A sense is a faculty by which outside stimuli are perceived. Many neurologists disagree about how many senses there actually are due to a broad interpretation of the definition of a sense. Our senses are split into two different groups. Our Exteroceptors detect stimulation from the outsides of our body. For example smell,taste,and equilibrium. The Interoceptors receive stimulation from the inside of our bodies. For instance, blood pressure dropping, changes in the gluclose and Ph levels. Children are generally taught that there are five senses (sight, hearing, touch, smell, taste). However, it is generally agreed that there are at least seven different senses in humans, and a minimum of two more observed in other organisms. Sense can also differ from one person to the next. Take taste for an example, what may taste great to me will taste awful to someone else. This all has to do with how our brains interpret the stimuli that is given. Chemoreception The senses of Gustation (taste) and Olfaction (smell) fall under the category of Chemoreception. Specialized cells act as receptors for certain chemical compounds. As these compounds react with the receptors, an impulse is sent to the brain and is registered as a certain taste or smell. Gustation and Olfaction are chemical senses because the receptors they contain are sensitive to the molecules in the food we eat, along with the air we breath. -

The Roles and Functions of Cutaneous Mechanoreceptors Kenneth O Johnson
455 The roles and functions of cutaneous mechanoreceptors Kenneth O Johnson Combined psychophysical and neurophysiological research has nerve ending that is sensitive to deformation in the resulted in a relatively complete picture of the neural mechanisms nanometer range. The layers function as a series of of tactile perception. The results support the idea that each of the mechanical filters to protect the extremely sensitive recep- four mechanoreceptive afferent systems innervating the hand tor from the very large, low-frequency stresses and strains serves a distinctly different perceptual function, and that tactile of ordinary manual labor. The Ruffini corpuscle, which is perception can be understood as the sum of these functions. located in the connective tissue of the dermis, is a rela- Furthermore, the receptors in each of those systems seem to be tively large spindle shaped structure tied into the local specialized for their assigned perceptual function. collagen matrix. It is, in this way, similar to the Golgi ten- don organ in muscle. Its association with connective tissue Addresses makes it selectively sensitive to skin stretch. Each of these Zanvyl Krieger Mind/Brain Institute, 338 Krieger Hall, receptor types and its role in perception is discussed below. The Johns Hopkins University, 3400 North Charles Street, Baltimore, MD 21218-2689, USA; e-mail: [email protected] During three decades of neurophysiological and combined Current Opinion in Neurobiology 2001, 11:455–461 psychophysical and neurophysiological studies, evidence has accumulated that links each of these afferent types to 0959-4388/01/$ — see front matter © 2001 Elsevier Science Ltd. All rights reserved. a distinctly different perceptual function and, furthermore, that shows that the receptors innervated by these afferents Abbreviations are specialized for their assigned functions. -

Auditory Experience Controls the Maturation of Song Discrimination and Sexual Response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi*
RESEARCH ARTICLE Auditory experience controls the maturation of song discrimination and sexual response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi* Graduate School of Science, Nagoya University, Nagoya, Japan Abstract In birds and higher mammals, auditory experience during development is critical to discriminate sound patterns in adulthood. However, the neural and molecular nature of this acquired ability remains elusive. In fruit flies, acoustic perception has been thought to be innate. Here we report, surprisingly, that auditory experience of a species-specific courtship song in developing Drosophila shapes adult song perception and resultant sexual behavior. Preferences in the song-response behaviors of both males and females were tuned by social acoustic exposure during development. We examined the molecular and cellular determinants of this social acoustic learning and found that GABA signaling acting on the GABAA receptor Rdl in the pC1 neurons, the integration node for courtship stimuli, regulated auditory tuning and sexual behavior. These findings demonstrate that maturation of auditory perception in flies is unexpectedly plastic and is acquired socially, providing a model to investigate how song learning regulates mating preference in insects. DOI: https://doi.org/10.7554/eLife.34348.001 Introduction Vocal learning in infants or juvenile birds relies heavily on the early experience of the adult conspe- cific sounds. In humans, early language input is necessary to form the ability of phonetic distinction *For correspondence: and pattern detection in the phase of auditory learning (Doupe and Kuhl, 1999; Kuhl, 2004). [email protected] Because of the strong parallels between speech acquisition of humans and song learning of song- Competing interests: The birds, and the difficulties to investigate the neural mechanisms of human early auditory memory at authors declare that no cellular resolution, songbirds have been used as a predominant model in studying memory formation competing interests exist. -

Center Surround Receptive Field Structure of Cone Bipolar Cells in Primate Retina
Vision Research 40 (2000) 1801–1811 www.elsevier.com/locate/visres Center surround receptive field structure of cone bipolar cells in primate retina Dennis Dacey a,*, Orin S. Packer a, Lisa Diller a, David Brainard b, Beth Peterson a, Barry Lee c a Department of Biological Structure, Uni6ersity of Washington, Box 357420, Seattle, WA 98195-7420, USA b Department of Psychology, Uni6ersity of California Santa Barbara, Santa Barbara, CA, USA c Max Planck Institute for Biophysical Chemistry, Gottingen, Germany Received 28 September 1999; received in revised form 5 January 2000 Abstract In non-mammalian vertebrates, retinal bipolar cells show center-surround receptive field organization. In mammals, recordings from bipolar cells are rare and have not revealed a clear surround. Here we report center-surround receptive fields of identified cone bipolar cells in the macaque monkey retina. In the peripheral retina, cone bipolar cell nuclei were labeled in vitro with diamidino-phenylindole (DAPI), targeted for recording under microscopic control, and anatomically identified by intracellular staining. Identified cells included ‘diffuse’ bipolar cells, which contact multiple cones, and ‘midget’ bipolar cells, which contact a single cone. Responses to flickering spots and annuli revealed a clear surround: both hyperpolarizing (OFF) and depolarizing (ON) cells responded with reversed polarity to annular stimuli. Center and surround dimensions were calculated for 12 bipolar cells from the spatial frequency response to drifting, sinusoidal luminance modulated gratings. The frequency response was bandpass and well fit by a difference of Gaussians receptive field model. Center diameters were all two to three times larger than known dendritic tree diameters for both diffuse and midget bipolar cells in the retinal periphery. -
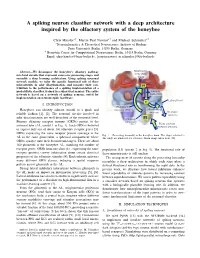
A Spiking Neuron Classifier Network with a Deep Architecture Inspired By
A spiking neuron classifier network with a deep architecture inspired by the olfactory system of the honeybee Chris Hausler¨ ∗†, Martin Paul Nawrot∗† and Michael Schmuker∗† ∗Neuroinformatics & Theoretical Neuroscience, Institute of Biology Freie Universitat¨ Berlin, 14195 Berlin, Germany † Bernstein Center for Computational Neuroscience Berlin, 10115 Berlin, Germany Email: [email protected], fmartin.nawrot, [email protected] Abstract—We decompose the honeybee’s olfactory pathway Mushroom into local circuits that represent successive processing stages and body (MB) calyx resemble a deep learning architecture. Using spiking neuronal network models, we infer the specific functional role of these microcircuits in odor discrimination, and measure their con- 2 tribution to the performance of a spiking implementation of a probabilistic classifier, trained in a supervised manner. The entire Kenyon network is based on a network of spiking neurons, suited for Cells (KC) implementation on neuromorphic hardware. Lateral horn 3 I. INTRODUCTION Projection Honeybees can identify odorant stimuli in a quick and neurons (PN) MB-extrinsic neurons To motor reliable fashion [1], [2]. The neuronal circuits involved in neurons odor discrimination are well described at the structural level. 1 Antennal Primary olfactory receptor neurons (ORNs) project to the lobe (AL) From odorant antennal lobe (AL, circuit 1 in Fig. 1). Each ORN is believed receptor neurons to express only one of about 160 olfactory receptor genes [3]. ORNs expressing the same receptor protein converge in the Fig. 1. Processing hierarchy in the honeybee brain. The stages relevant to AL in the same glomerulus, a spherical compartment where this study are numbered for reference. -

Sensory Receptors
Laboratory Worksheet Exercise: Sensory Receptors Sense Organs - Sensory Receptors A sensory receptor is a specialized ending of a sensory neuron that detects a specific stimulus. Receptors can range from simple nerve endings of a sensory neuron (e.g., pain, touch), to a complex combination of nervous, epithelial, connective and muscular tissue (e.g., the eyes). Axon Synaptic info. Sensory end bulbs Receptors Figure 1. Diagram of a sensory neuron with sensory information being detected by sensory receptors located at the incoming end of the neuron. This information travels along the axon and delivers its signal to the central nervous system (CNS) via the synaptic end bulbs with the release of neurotransmitters. The function of a sensory receptor is to act as a transducer. Transducers convert one form of energy into another. In the human body, sensory receptors convert stimulus energy into electrical impulses called action potentials. The frequency and duration of action potential firing gives meaning to the information coming in from a specific receptor. The nervous system helps to maintain homeostasis in the body by monitoring the internal and external environments of the body using receptors to achieve this. Sensations are things in our environment that we detect with our 5 senses. The 5 basic senses are: Sight Hearing Touch Taste Smell An adequate stimulus is a particular form of energy to which a receptor is most responsive. For example, thermoreceptors are more sensitive to temperature than to pressure. The threshold of a receptor is the minimum stimulus required to activate that receptor. Information about Receptor Transmission Sensory receptors transmit four kinds of information - modality, location, intensity and duration. -

The Visual System: Higher Visual Processing
The Visual System: Higher Visual Processing Primary visual cortex The primary visual cortex is located in the occipital cortex. It receives visual information exclusively from the contralateral hemifield, which is topographically represented and wherein the fovea is granted an extended representation. Like most cortical areas, primary visual cortex consists of six layers. It also contains, however, a prominent stripe of white matter in its layer 4 - the stripe of Gennari - consisting of the myelinated axons of the lateral geniculate nucleus neurons. For this reason, the primary visual cortex is also referred to as the striate cortex. The LGN projections to the primary visual cortex are segregated. The axons of the cells from the magnocellular layers terminate principally within sublamina 4Ca, and those from the parvocellular layers terminate principally within sublamina 4Cb. Ocular dominance columns The inputs from the two eyes also are segregated within layer 4 of primary visual cortex and form alternating ocular dominance columns. Alternating ocular dominance columns can be visualized with autoradiography after injecting radiolabeled amino acids into one eye that are transported transynaptically from the retina. Although the neurons in layer 4 are monocular, neurons in the other layers of the same column combine signals from the two eyes, but their activation has the same ocular preference. Bringing together the inputs from the two eyes at the level of the striate cortex provide a basis for stereopsis, the sensation of depth perception provided by binocular disparity, i.e., when an image falls on non-corresponding parts of the two retinas. Some neurons respond to disparities beyond the plane of fixation (far cells), while others respond to disparities in front of the plane of the fixation (near cells). -

Branched Cells in the Epidermis: an Overview
0022-202X/ 80/ 7501-0006$02.00/0 THE JOURNAL OF INVESTIGATIVE D ERMATOLOGY, 75:6-11, 1980 Vol. 75, No.1 Copyrighl © 1980 by The Williams & Wilkins Co. Printed in U.S.A. Branched Cells in the Epidermis: An Overview A. S. BREATHNACH, M.Sc., M.D. Department of Anatomy, St. Mary's Hospital Medical School (University of London), London, W.2, UK Current knowledge concerning the nature, lineage, due, no doubt, to overconcentration upon their own pet clear and function of the Langerhans cell, Merkel cell, and, to cells, to the fact that no specific stains for lymphocytes had a lesser extent, the melanocyte, are reviewed under been evolved, to the unacceptability of Andrew's conclusions headings that emphasize the confederate constitution of [ 4] that lymphocytes were capable of differentiating into prac the epidermis as a compound tissue composed of a vari tically every other variety of epidermal cell, and finally to the ety of cellular elements; the role of the lymphocyte as a lack of an obvious reason for their presence there at all under component of normal epidermis is also considered. It normal circumstances. appears that the function of the Langerhans cell has Today; a further 20 yr along the way, this question of intrae finally been established, i.e., it serves as a front-line pidermallymphocytes has become an issue of vital importance, element in immune reactions of the skin. Develop not only in relation to individual immunopathologic situations, mentally, it is of mesenchymal origin. The Merkel cell but also from the wider points of view put forward by Fichthel still presents a number of problems centering around ius, Groth, and Liden [5] and Streilein [6] in connection with questions of its lineage, the nature of its characteristic the skin as an immunologic inductive microenvironment, and granules, and the "synaptic" relationship between it and the possible existence of a subpopulation of lymphocytes sub the associated neurite. -

1P MON. PM Cortex and Subcortical Auditory Nuclei
dynamic role for the vestibular system in orientation and flight control. Laboratory studies of flight behavior under illuminated and dark conditions in both static and rotating obstacle tests were carried out while administering heavy water ͑D2O͒ to bats to impair their vestibular inputs. Eptesicus carried out complex maneuvers through both fixed arrays of wires and a rotating obstacle array using both vision and echolocation, or when guided by echolocation alone. When treated with D2O in combination with lack of visual cues, bats showed considerable decrements in performance. These data indicate that big brown bats use both vision and echolocation to provide spatial registration for head position information generated by the vestibular system. 2:55 1pAB3. Bat’s auditory system: Corticofugal feedback and plasticity. Nobuo Suga ͑Dept. of Biol., Washington Univ., One Brookings Dr., St. Louis, MO 63130͒ The auditory system of the mustached bat consists of physiologically distinct subdivisions for processing different types of biosonar information. It was found that the corticofugal ͑descending͒ auditory system plays an important role in improving and adjusting auditory signal processing. Repetitive acoustic stimulation, cortical electrical stimulation or auditory fear conditioning evokes plastic changes of the central auditory system. The changes are based upon egocentric selection evoked by focused positive feedback associated with lateral inhibition. Focal electric stimulation of the auditory cortex evokes short-term changes in the auditory 1p MON. PM cortex and subcortical auditory nuclei. An increase in a cortical acetylcholine level during the electric stimulation changes the cortical changes from short-term to long-term. There are two types of plastic changes ͑reorganizations͒: centripetal best frequency shifts for expanded reorganization of a neural frequency map and centrifugal best frequency shifts for compressed reorganization of the map. -

Somatosensory Function in Speech Perception
Somatosensory function in speech perception Takayuki Itoa, Mark Tiedea,b, and David J. Ostrya,c,1 aHaskins Laboratories, 300 George Street, New Haven, CT 06511; bResearch Laboratory of Electronics, Massachusetts Institute of Technology, 50 Vassar Street, Cambridge, MA 02139; and cDepartment of Psychology, McGill University, 1205 Dr. Penfield Avenue, Montreal, QC, Canada H3A 1B1 Edited by Fernando Nottebohm, The Rockefeller University, Millbrook, NY, and approved December 4, 2008 (received for review October 7, 2008) Somatosensory signals from the facial skin and muscles of the vocal taken from a computer-generated continuum between the words tract provide a rich source of sensory input in speech production. head and had. We found that perception of these speech sounds We show here that the somatosensory system is also involved in varied in a systematic manner depending on the direction of skin the perception of speech. We use a robotic device to create stretch. The presence of any perceptual change at all depended patterns of facial skin deformation that would normally accom- on the temporal pattern of the stretch, such that perceptual pany speech production. We find that when we stretch the facial change was present only when the timing of skin stretch was skin while people listen to words, it alters the sounds they hear. comparable to that which occurs during speech production. The The systematic perceptual variation we observe in conjunction findings are consistent with the hypothesis that the somatosen- with speech-like patterns of skin stretch indicates that somatosen- sory system is involved in the perceptual processing of speech. sory inputs affect the neural processing of speech sounds and The findings underscore the idea that there is a broad nonau- shows the involvement of the somatosensory system in the per- ditory basis to speech perception (17–21).