Chemistry and Sport
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Etn1956 Vol02 21
TRACK NEwSL TER Vol. 2, No. 21, June 19, 1956 P.O. Box 296, Los Altos, Calif. By Bert & Cordner Nelson, Track & F'ield News $6 per year (24 issues) NEWS NCAA, Berkeley, June 15-16: 100- Morrow 10.4 (a gainst wind), Sime 10.55,. \.___,, Agostini . 10.55, Kin g 10, 6 , Kave10.6, Blair 10.7; 200-Morrow 20.6 turn; e quals be st ev er, Blair 21. 0 , Whi l de n 21. 2, Ago st i ri"l21 . 2 , Brabham r 2 1. 4., Se grest 21 .5. ( Sime pulled u p lame); 1-1-00-Ma shbu rn 46.4, Ha i nes 46.4, Jenkins 46 . 6 , Ellis46.7, Wash i n gton 47:T, Pe r kins 47._,2; 800 - Sowell 1:4 6 .7, American record, Sta nl ey 1:4 9 .2, Brew 1:50.5, Johnson 1: 50 . 5 , Had l ey 1: 5 1.1, Jan zen 1:52. 9 (Kirkby 3rd 1: 50 . 2 but disquali fi ed ); 1500 - Delany 3 :1.~7.3 (54 .1 last l.1_L~0), Bai l ey 3:47. 5 , Wing 3:Li.9 .7 ,. Sean1an 'JT[f9'.7, Whee l er J :50. 4 , :Murphey J:52.0; J OOOSC-Kennedy 9 :1 6 ,5., Matza 9 :17.2, Kielstru p 9 : 34 -4 , Hubbard 9 :42 .7, Peterson 9 :46 .1, · Fergus on 10:01.1; 5000-Delli ng er 14: 48 .5, Beatty 14 : 51 ,1, Jones 14: 52 .2, Truex l LJ.: 53 .5, Wallin gford ll+:53.7, Shim 15 :0L~.14-; 10,000 (F'riday ; J ones 31 :15.3, House 31:4.6 , Sbarra 32: 0l , Frame 32 : 24 .7, McNeal · 32:42.6, McClenathen 33:13,0; ll OI:I-Calhoun 13.7, J ohnson 13 . -

3677 Hon. Dennis J. Kucinich Hon. Joe Baca
March 14, 2001 EXTENSIONS OF REMARKS 3677 seeking care at more than 40 types of out- TRIBUTE TO LEAMON KING The Delano Record dated May 15, 1956 patient settings. The office-based surgery stated the following: ‘‘King’s 9.3 Dash Brings standards were established specifically for sin- HON. JOE BACA Another Record to City. Delano became the gle sites of care with up to four physicians, OF CALIFORNIA home of two world champions Saturday when dentists or podiatrists. IN THE HOUSE OF REPRESENTATIVES Leamon King, local resident and former Dela- no High School track star, ran the 100 yards JCAHO evaluates and accredits nearly Wednesday, March 14, 2001 dash in 9.3 at the Fresno Relays to tie the 19,000 health care organizations and pro- Mr. BACA. Mr. Speaker, I would like to sa- world record. King’s victory brought another grams in the United States. Accreditation is lute Leamon King, of California. Leamon has world record to Delano, making it the home of recognized nationwide as a symbol of quality been recognized by Adelante, California Mi- one the fastest sprinters and the residence of that indicates that an organization meets cer- grant Leadership Council and American Le- Lon Spurrier, holder of the world record for the tain performance standards. JCAHO has cer- gion Merle Reed Post 124 as an outstanding 880. There is no city in the United States the tainly chosen a good place to start its accredi- individual who has made significant contribu- size of Delano, which can boast two world tation program of office-based surgery by tions to the improvement of education opportu- champions.’’ starting in Salinas. -

Hall of Fame
scottishathletics HALL OF FAME 2018 October A scottishathletics history publication Hall of Fame 1 Date: CONTENTS Introduction 2 Jim Alder, Rosemary Chrimes, Duncan Clark 3 Dale Greig, Wyndham Halswelle 4 Eric Liddell 5 Liz McColgan, Lee McConnell 6 Tom McKean, Angela Mudge 7 Yvonne Murray, Tom Nicolson 8 Geoff Parsons, Alan Paterson 9 Donald Ritchie, Margaret Ritchie 10 Ian Stewart, Lachie Stewart 11 Rosemary Stirling, Allan Wells 12 James Wilson, Duncan Wright 13 Cover photo – Allan Wells and Patricia Russell, the daughter of Eric Liddell, presented with their Hall of Fame awards as the first inductees into the scottishathletics Hall of Fame (photo credit: Gordon Gillespie). Hall of Fame 1 INTRODUCTION The scottishathletics Hall of Fame was launched at the Track and Field Championships in August 2005. Olympic gold medallists Allan Wells and Eric Liddell were the inaugural inductees to the scottishathletics Hall of Fame. Wells, the 1980 Olympic 100 metres gold medallist, was there in person to accept the award, as was Patricia Russell, the daughter of Liddell, whose triumph in the 400 metres at the 1924 Olympic Games was an inspiration behind the Oscar-winning film Chariots of Fire. The legendary duo were nominated by a specially-appointed panel consisting of Andy Vince, Joan Watt and Bill Walker of scottishathletics, Mark Hollinshead, Managing Director of Sunday Mail and an on-line poll conducted via the scottishathletics website. The on-line poll resulted in the following votes: 31% voting for Allan Wells, 24% for Eric Liddell and 19% for Liz McColgan. Liz was inducted into the Hall of Fame the following year, along with the Olympic gold medallist Wyndham Halswelle. -

NEWSLETTER Supplementingtrack & FIELD NEWS Twice Monthly
TRACKNEWSLETTER SupplementingTRACK & FIELD NEWS twice monthly. Vol. 10, No. 1 August 14, 1963 Page 1 Jordan Shuffles Team vs. Germany British See 16'10 1-4" by Pennel Hannover, Germany, July 31- ~Aug. 1- -Coach Payton Jordan London, August 3 & 5--John Pennel personally raised the shuffled his personnel around for the dual meet with West Germany, world pole vault record for the fifth time this season to 16'10¼" (he and came up with a team that carried the same two athletes that com has tied it once), as he and his U.S. teammates scored 120 points peted against the Russians in only six of the 21 events--high hurdles, to beat Great Britain by 29 points . The British athl_etes held the walk, high jump, broad jump, pole vault, and javelin throw. His U.S. Americans to 13 firsts and seven 1-2 sweeps. team proceeded to roll up 18 first places, nine 1-2 sweeps, and a The most significant U.S. defeat came in the 440 relay, as 141 to 82 triumph. the Jones boys and Peter Radford combined to run 40 . 0, which equal The closest inter-team race was in the steeplechase, where ed the world record for two turns. Again slowed by poor baton ex both Pat Traynor and Ludwig Mueller were docked in 8: 44. 4 changes, Bob Hayes gained up to five yards in the final leg but the although the U.S. athlete was given the victory. It was Traynor's U.S. still lost by a tenth. Although the American team had hoped second fastest time of the season, topped only by his mark against for a world record, the British victory was not totally unexpected. -

Mrnmmmmmmjmk*' Cheyney G.F.Pts
THE SUNDAY STAR, Washington, D. C. ** SUNDAY, JANUARY 10, I»A4 C-3 Field for Star Games Bolstered by Flood of New York Talent ? Lack of Any Conflict TV Board Renamed, Golden Gloves Entries Byrd Guest Speaker Jan. 23 Makes D. C. gfl w Emphasizing NCAA's Indicate Battle tor At Home Plate Event Dr. H C. (Curley) Byrd, presi- Track World Capital Stand-Pat Policy Heavyweight Honors & beat dent emeritus of Maryland Uni- « versity, guest By BURTON HAWKINS will be speaker at By Rod Thomas By Ik Auociatad Pratt Early entry lists indicate the the third annual banquet of the For 35 years, as boy man, CINCINNATI. Jan. 9.—The accent will be heavyweights and on For the first time since he be- and Dick Kokos of the Orioles, Dorsey J. Griffith, the old George- Council of the National Collegi- Home Plate Club at 7:30 pm. and light-heavyweights in the came affiliated with the Sena- . Any significance in the fact town sprinter, steeped ate Athletic Association reap- | Saturday at the National Press has been boxing years ago. that five of the eight are in track and field. Few men pointed its television committee Golden Gloves tourna- tors more than 40 have Clark Griffith won’t stay at the pitchers? Club. so today with only one change. ment opening January 19 at been closely same hotel Baltimore officials apparently Also expected to attend are identified with Wilbur V. Hubbard of San Turner’s Arena. Jose State College replaces M. I. no longer are proud of their pre- Senator Johnson of Colorado, Seldom before in the history ! . -

UNA Executives Activate Tax-Exempt, Non-Profit Foundation Kuchma Lauds
INSIDE:• The Ukrainian language’s prospects for the future — page 2. • Black Sea Fleet still a sticking point in Ukrainian-Russian relations — page 3. • More exclusive coverage of the Summer Olympiad — pages 8-13. Published by the Ukrainian National Association Inc., a fraternal non-profit association Vol. LXIV HE No.KRAINIAN 32 THE UKRAINIAN WEEKLY SUNDAY, AUGUST 11, 1996 EEKLY$1.25/$2 in Ukraine OlympiansT rest onU their laurels at closing ceremonyW of Summer Games Ukraine makes top 10 in medal count by Roman Woronowycz count, placing in that position with 23 medals, ahead of nations like Canada, ATLANTA — At the opening cere- Britain, Poland and Brazil. Ukraine took monies and in the 16 days since, they nine gold medals, highest over all. were the center of attention. On August 4 Minister of Youth and Sports Valeriy the athletes of the XXVI Summer Games Borzov almost hit the mark when he, could sit on their laurels, for one night at albeit reluctantly, predicted during an least, and enjoy the closing ceremonies. interview at The Weekly in February that Many of the Ukrainian athletes, those Ukraine would take 10 gold medals. who had completed their competitions by Sure, Ukraine experienced some fail- the previous Wednesday, had left by ures — Sergey Bubka’s withdrawal from chartered jet on Thursday, ostensibly the pole vault competition because of because the National Olympic problems with his Achilles’ heel (had he Committee of Ukraine is trying to keep not been injured that might have been the costs down. The ones who remained had 10th gold that Mr. -

Payton Jordan.Pdf
p.1 STANFORD UNIVERSITY PROJECT: Bob Murphy Interviews INTERVIEWEE: Payton Jordan Robert W. Murphy, Jr.: [0:00] Hello again everybody, Bob Murphy here and a very special chapter in Stanford sports today because one of the dearest friend I've ever had in my life and one of my great pals, Payton Jordan, is with us. Payton, this was scheduled long before you hit your little speed bump a week or so ago. So we'll tell the folks about that, but as we start doing this, I think of you and I sharing the better part of the last 50 years telling stories to one another. Laughing with one another. Laughing at one another. [laughter] Murphy: [0:38] But here we are to recap this. Tell the folks about your little speed bump, you're doing fine, you look great, things are gonna be fine. Payton Jordan: [0:46] I'm sure everything will be fine, I had a slight bump in road, had a little lump on my neck. And they found out it was a very rare cancer and we had to do a little cutting and we'll be doing some radiation and in no time at all, I'll be back up and at them. Murphy: [1:00] They didn't give you a face lift, too, because you're looking so pretty here. [both laugh] Jordan: [1:05] They kind of knit my nerves on one side a little bit, but I'm going to be OK. Murphy: [1:09] We're going to have fun talking about this, we're in no hurry, we're just gonna kind of ramble on. -

Amateurism and Coaching Traditions in Twentieth Century British Sport
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by E-space: Manchester Metropolitan University's Research Repository Uneasy Bedfellows: Amateurism and Coaching Traditions in Twentieth Century British Sport Tegan Laura Carpenter A thesis submitted in partial fulfilment of the requirements of the Manchester Metropolitan University for the degree of Doctor of Philosophy July 2012 Tegan Carpenter July 2012 If you do well in sport and you train, ‘good show’, but if you do well in sport and you don’t train, ‘bloody good show’. Geoffrey Dyson, 1970 Tegan Carpenter July 2012 Dedication This thesis is proudly dedicated to my parents, Lynne and John, my two brothers, Dan and Will and my best friend, Steve - Thank you for always believing in me. Tegan Carpenter July 2012 Acknowledgments This thesis would not have been possible without the continued support of family, friends and colleagues. While I am unable to acknowledge you all individually - I will be forever indebted to you. To my supervisor, Dr Dave Day - I consider myself incredibly lucky to have had such an attentive and committed mentor. Someone who transformed the trudge of a PhD into an enjoyable journey, and because of this, I would not hesitate accepting the opportunity again (even after knowing the level of commitment required!). Thank you for never losing faith in me and for your constant support and patience along the way. I would also like to thank Dr Neil Carter and Professor Martin Hewitt for their comments and advice. Special thanks to Sam for being the best office buddy and allowing me to vent whenever necessary! To Margaret and the interviewees of this study – thank you for your input and donating your time. -

Etn1959 Vol06 10
' / \ '_. k I ~ / l f RACKNEWSL£1TE r . ".' , also KV\ownas - [1R~tlf N'1ts1~trERI , , I _/_ sJ , (OFFlCl~L P\.l8L\C/\TION Or l'RKK NUiS OF i11E 'WO~\.0J\lN1t.1c) Vol. 6, No, 10, Dec. 23, 1959 Semi-Monthly --$6per y~ar by first class tbaH_. NEWS METROPOLITAN AAU FIELD EVENT MEET, New York City, Dec, 5: 35 lb. wt. throw, Engel (NYPC) 63'8½" (scratch); SP, D'Atnico (Manhattan frosh) 56'7~" (six-foot handicap), Marchiony (Manhattan) 56'7¼" (1'10" handicap); BJ, McBride (Manhattan) i4'2" --(11 811 hanaicap); PV, , Barr (St. John's) 14'9" (1'9'' handicap). ' , , STANFORD ALL-COMERS, (all SCYG unless noted}: Dec. 12:-1320, Sargent 3: 07. 6; Curtis 3:08. 7; McGee, 3:14. 7. Dec. 19: 3,000 meters, 40 yards: Beatty 8:36. 3; Kelly 8:43. O; Bishop 8:44. ' 0; Sargent 8: 50. 3; McGee 8:53. O; l0Oy, Thomason 10. 2; 660, Toomey (Colo _,_) 1:22.2, Mccalla (Berkeley H.S.) 1:26.0. " ' WESTERN HEMISPHERE MARATHON: Culver City, Calif. Dec. 12: Torn Ryan (Culver City A.C.) 2:28:30, new ·course record. Old record / 2:32:35.4, Allan, 1958. SOUTH AF RICA: Sasolburg, Nov., 25: l00y, Gamper (GerrhaIJ.y) 9. 9; -440, P.otgiefu:,;, 48. 2; mile; Brenner (Gerrnruty) 4d2. 2, Clark 4:14. 3; 220LH, Pqtgieter 23. 6; SP, Wegmann · -(Germany) 56'5½"; DT, du Plessis 178'2". Pretoria, Nov. 28: l0Oy, Bromberg 9.5, Jefferys 9. 5, Luxon 9. 6, Gamper 9. 7; 440, Spence 46. -

The Life and Times of Herb Mckenley
iHI:;�LEANER. SATURDAY, DECEMBER 8, 2007 I HERB MCKENLi::Y FEATURE ;. ·. [ · . -� ·;_. �- , Extracts fl· . 'O"-.< .....,.. _ lit McKenley :·.· . 1ary 'l_� �� t�� ... • -,.�-. -�.·- ..._, � ' July 10, 1922: I was born a George Rhoden and 1 --WM-f.\a\te: ' fl'f$ftJme. Not only does he win, short while ago in Pleasant Valley, qualified for the seml:ff�V�t\�t�� �at'lie equalled the Olympic a small village in Clarendon. My the 400 metres in the· . ·�"''-· _: !'eCOrO. 1 won the silver. ,, parents are Dr. Alexander Games taking place .at · .:t?!� ·} � .., McKenley and his wife, Zilpha. London. We are so prowd. aMt .August 6, 1948: The Jamaica · :';" hopeful. A year earller."l ha'a eel� ' team of Arthur Wint, George April 6, 1938: Competing for ebrated getting one of my 'first· Rhoden, Basil McKenzie and I is Calabar High School, I came sec awards, the Athlete of theYear: • vtctorlous in the Olympic relay ond In the 220 yards Class Two for Central and South Jtrnerica, 1,600 metres semi-finals on race at the Inter-Secondary after setting a world-record 46.3 what is a rain-soaked track. We Schools Championship Sports seconds. 1 was the first Jamaican did so with the greatest ease, held at Sabina Park in Kingston. It to set a time or distance meas- beating France and Canada who Is the only time 1 placed in an ured world record In any sport. are our main rivals. event In these sports. I was beat en by LB. Jones, also of Calabar. August 5, 1948: It i_s·Arthur August 7, 1948: A cramp Third place was won by Wilson Wlnt who won the gold medal seized Arthur Wlnt after 150 Chung of St. -

All Time Men's World Ranking Leader
All Time Men’s World Ranking Leader EVER WONDER WHO the overall best performers have been in our authoritative World Rankings for men, which began with the 1947 season? Stats Editor Jim Rorick has pulled together all kinds of numbers for you, scoring the annual Top 10s on a 10-9-8-7-6-5-4-3-2-1 basis. First, in a by-event compilation, you’ll find the leaders in the categories of Most Points, Most Rankings, Most No. 1s and The Top U.S. Scorers (in the World Rankings, not the U.S. Rankings). Following that are the stats on an all-events basis. All the data is as of the end of the 2019 season, including a significant number of recastings based on the many retests that were carried out on old samples and resulted in doping positives. (as of April 13, 2020) Event-By-Event Tabulations 100 METERS Most Points 1. Carl Lewis 123; 2. Asafa Powell 98; 3. Linford Christie 93; 4. Justin Gatlin 90; 5. Usain Bolt 85; 6. Maurice Greene 69; 7. Dennis Mitchell 65; 8. Frank Fredericks 61; 9. Calvin Smith 58; 10. Valeriy Borzov 57. Most Rankings 1. Lewis 16; 2. Powell 13; 3. Christie 12; 4. tie, Fredericks, Gatlin, Mitchell & Smith 10. Consecutive—Lewis 15. Most No. 1s 1. Lewis 6; 2. tie, Bolt & Greene 5; 4. Gatlin 4; 5. tie, Bob Hayes & Bobby Morrow 3. Consecutive—Greene & Lewis 5. 200 METERS Most Points 1. Frank Fredericks 105; 2. Usain Bolt 103; 3. Pietro Mennea 87; 4. Michael Johnson 81; 5. -
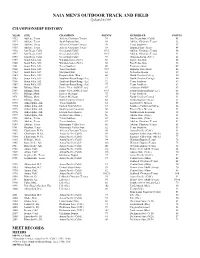
NAIA MEN's OUTDOOR TRACK and FIELD Updated 6/1/09
NAIA MEN'S OUTDOOR TRACK AND FIELD Updated 6/1/09 CHAMPIONSHIP HISTORY YEAR SITE CHAMPION POINTS RUNNER-UP POINTS 1952 Abilene, Texas Abilene Christian (Texas) 74 San Diego State (Calif.) 45 1953 Abilene, Texas South Dakota State 48 Abilene Christian (Texas) 42 1954 Abilene, Texas Abilene Christian (Texas) 39 Texas Southern 33 1955 Abilene, Texas Abilene Christian (Texas) 68 Emporia State (Kan.) 44 1956 San Diego, Calif. Occidental (Calif.) 89.5 Abilene Christian (Texas) 56 1957 San Diego, Calif. Occidental (Calif.) 148.5 Abilene Christian (Texas) 34 1958 San Diego, Calif. Occidental (Calif.) 93 Winston-Salem (N.C.) 62 1959 Sioux Falls, S.D. Winston-Salem (N.C.) 56 East Texas State 55 1960 Sioux Falls, S.D. Winston-Salem (N.C.) 58 East Texas State 45 1961 Sioux Falls, S.D. Texas Southern 49 Tennessee State 47 1962 Sioux Falls, S.D. Texas Southern 72.5 Emporia State (Kan.) 46 1963 Sioux Falls, S.D. Maryland State 82 Nebraska-Omaha 33 1964 Sioux Falls, S.D. Emporia State (Kan.) 60 North Carolina College 50 1965 Sioux Falls, S.D. Southern-Baton Rouge (La.) 77 North Carolina College 40 1966 Sioux Falls, S.D. Southern-Baton Rouge (La.) 92 Texas Southern 69 1967 Sioux Falls, S.D. Southern-Baton Rouge (La.) 77 Texas Southern 63 1968 Billings, Mont. Prairie View A&M (Texas) 47 Arkansas AM&N 45 1969 Billings, Mont. Prairie View A&M (Texas) 69.5 Southern-Baton Rouge (La.) 68 1970 Billings, Mont. Eastern Michigan 75 Texas Southern 50 1971 Billings, Mont. Eastern Michigan 65 North Carolina Central 43 1972 Billings, Mont.