Suxamethonium Chloride in Each Ml of Water for Injections BP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Study of Different Doses of Rocuronium Bromide For
MedDocs Publishers Annals of Anesthesia and Pain Medicine Open Access | Research Article Comparative study of different doses of rocuronium bromide for endotracheal intubation Nidhi V Sardhara1*; Sonal A Shah2; Dhaval P Pipaliya3; Shivansh Gupta3 1SVP hospital, 13th floor, Room no 13125, Near Ellis bridge, Ahmedabad, Gujarat-380006 2Assistant Professor, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India 3First year Resident, Department of anesthesia, Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India *Corresponding Author(s): Nidhi V Sardhara Abstract SVP hospital, 13th floor, Room no 13125, Near Ellis Background: Endotracheal intubation is one of such bridge, Ahmedabad, Gujarat-380006 development without which general anesthesia cannot be Tel: +91-9428-44-7878, Fax: 7926-57-8452 considered safe for any major surgery particularly head and Email: [email protected] neck, thoracic and abdominal surgeries. Ever since the ad- vent of anesthesia, anesthesiologists have been in search of an ideal muscle relaxants which can provide ideal intu- bating conditions in ultrashort duration with minimal side effects. Rocuronium bromide provides fast onset of action, Received: Mar 11, 2020 an intermediate duration of action and rapid recovery, good Accepted: Apr 24, 2020 to excellent intubating conditions at doses having minimal Published Online: Apr 30, 2020 or no haemodynamic changes. Present study is to compare the effect of different doses (0.6 mg/kg, 0.9 mg/kg, 1.2 mg/ Journal: Annals of Anesthesia and Pain Medicine kg) of Rocuronium bromide for endotracheal intubation at Publisher: MedDocs Publishers LLC 60 seconds. Online edition: http://meddocsonline.org/ Material and methods: This study was carried out by Copyright: © Sardhara NV (2020). -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -
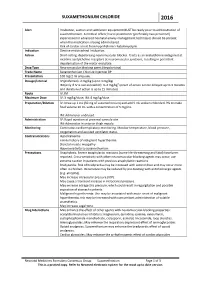
Suxamethonium Chloride 2016
SUXAMETHONIUM CHLORIDE 2016 Alert Intubation, suction and ventilation equipment MUST be ready prior to administration of suxamethonium. A medical officer/nurse practitioner (preferably two personnel) experienced in advanced neonatal airway management techniques should be present when the medication is being administered. Risk of cardiac arrest from hyperkalemic rhabdomyolysis Indication Elective endotracheal intubation. Action Short-acting, depolarising neuromuscular blocker. It acts as an acetylcholine antagonist at nicotinic acetylcholine receptors at neuromuscular junctions, resulting in persistent depolarisation of the motor end plate. Drug Type Neuromuscular blocking agent (depolarising) Trade Name Suxamethonium Chloride Injection BP Presentation 100 mg/2 ml ampoule. Dosage/Interval IV ( preferred): 2 mg/kg (up to 3 mg/kg) IM (only if IV is not accessible): 3–4 mg/kg9 (onset of action can be delayed up to 3 minutes and duration of action is up to 15 minutes) Route IV, IM Maximum Dose IV: 3 mg/kg/dose; IM: 4 mg/kg/dose Preparation/Dilution IV: Draw up 1 mL (50 mg of suxamethonium) and add 9 mL sodium chloride 0.9% to make final volume 10 mL with a concentration of 5 mg/mL. IM: Administer undiluted. Administration IV: Rapid injection at proximal cannula site. IM: Administer in anterior thigh muscle. Monitoring Continuous cardiorespiratory monitoring. Monitor temperature, blood pressure, oxygenation and assisted ventilator status. Contraindications Hyperkalaemia Family history of malignant hyperthermia Skeletal muscle myopathy Hypersensitivity to suxamethonium Precautions Anaphylaxis: Severe anaphylactic reactions (some life-threatening and fatal) have been reported. Cross-sensitivity with other neuromuscular-blocking agents may occur; use extreme caution in patients with previous anaphylactic reactions. -

Cardiovascular Monitoring with Acetylcholinesterase Inhibitors: a Clinical Protocol† Jeremy P
Advances in Psychiatric Treatment (2007), vol. 13, 178–184 doi: 10.1192/apt.bp.106.002725 Cardiovascular monitoring with acetylcholinesterase inhibitors: a clinical protocol† Jeremy P. Rowland, John Rigby, Adam C. Harper & Rosalind Rowland Abstract There has been significant anxiety among prescribers regarding the potential for cardiac adverse effects associated with acetylcholinesterase (AChE) inhibitors in Alzheimer’s disease. There is no consensus on how to manage this cardiovascular risk, and memory clinics vary widely in their practice. Review of published evidence reveals that the incidence of cardiovascular side-effects is low, and that serious adverse events are rare. Intensive cardiovascular screening such as pre-treatment electrocardiograms or 24 h cardiac monitoring is not justified. Furthermore, there are no high-risk groups to target. This article suggests pragmatic guidelines for managing cardiovascular risk in patients receiving AChE inhibitors. The guidelines are intended to be easy to incorporate into routine clinical practice in a memory clinic. A few years ago it was estimated that almost 18 thus followed some uncertainty as to how treatment million people worldwide had dementia (Alzheimer’s should be properly managed. Society, 2004), with Alzheimer’s disease accounting Since the manufacturers’ cautions remain (see the for over half of cases (Fratiglioni, 2000). The second- relevant entries in http://emc.medicines.org.uk) and generation acetylcholinesterase (AChE) inhibitors guidance has been unforthcoming, services now vary donepezil, rivastigmine and galantamine were widely in their practice: some, for example, require introduced into clinical practice from 1997 for the electrocardiograms (ECGs) before use and during symptomatic treatment of Alzheimer’s disease, and treatment, whereas others do not. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Mechanism of Central Hypopnoea Induced by Organic Phosphorus
www.nature.com/scientificreports OPEN Mechanism of central hypopnoea induced by organic phosphorus poisoning Kazuhito Nomura*, Eichi Narimatsu, Hiroyuki Inoue, Ryoko Kyan, Keigo Sawamoto, Shuji Uemura, Ryuichiro Kakizaki & Keisuke Harada Whether central apnoea or hypopnoea can be induced by organophosphorus poisoning remains unknown to date. By using the acute brainstem slice method and multi-electrode array system, we established a paraoxon (a typical acetylcholinesterase inhibitor) poisoning model to investigate the time-dependent changes in respiratory burst amplitudes of the pre-Bötzinger complex (respiratory rhythm generator). We then determined whether pralidoxime or atropine, which are antidotes of paraoxon, could counteract the efects of paraoxon. Herein, we showed that paraoxon signifcantly decreased the respiratory burst amplitude of the pre-Bötzinger complex (p < 0.05). Moreover, pralidoxime and atropine could suppress the decrease in amplitude by paraoxon (p < 0.05). Paraoxon directly impaired the pre-Bötzinger complex, and the fndings implied that this impairment caused central apnoea or hypopnoea. Pralidoxime and atropine could therapeutically attenuate the impairment. This study is the frst to prove the usefulness of the multi-electrode array method for electrophysiological and toxicological studies in the mammalian brainstem. Te pre-Bötzinger complex (preBötC) in the ventrolateral lower brainstem is essential for the formation of the unconscious breathing rhythm in mammals1,2. Tis is because the cyclic burst excitation generated from preBötC synchronizes with the respiratory rhythm through phrenic nerve fring and the diaphragmatic contractions, and destruction of preBötC causes the disappearance of the rhythm. Periodic respiratory burst excitation has also been confrmed from an island specimen derived by isolating preBötC in an island shape to block input from other neurons2. -

2020 Welldyne Formulary.Pdf
LEGEND TIER DESCRIPTION 1 Preferred Generics 2 Non-Preferred Generics 3 Preferred Brands 4 Non-Preferred Brands 5 Preferred Specialty 6 Non-Preferred Specialty TYPE DESCRIPTION There is a limit on the amount of this drug that is covered per QL Quantity Limit prescription, or within a specific time frame. You (or your physician) are required to get prior authorization before PA Prior Authorization you fill your prescription for this drug. Without prior approval, we may not cover this drug. In some cases, you may be required to first try certain drugs to treat ST Step Therapy your medical condition before we will cover another drug for that condition. This prescription drug may only be covered if you meet the minimum AL1 Age Limit or maximum age limit. LA Limited Access This prescription drug is limited to certain pharmacies. Specialty drugs are high-cost drugs used to treat complex or rare S Specialty Drug conditions, such as multiple sclerosis, rheumatoid arthritis, hepatitis C, and hemophilia. PAGE 1 LAST UPDATED 01/2020 LIST OF COVERED PRESCRIPTION MEDICATIONS PRODUCT DESCRIPTION TIER LIMITS & RESTRICTIONS ADHD/ANTI-NARCOLEPSY /ANTI-OBESITY/ANOREXIANT AGENTS ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS ADDERALL 4 AL1 Up to 25 yrs old ADDERALL XR 4 AL1 Up to 25 yrs old QL 30 / 30 day(s) ADHANSIA XR 4 AL1 Up to 25 yrs old QL 30 / 30 Days ADIPEX-P 4 PA QL 300 / 30 day(s) ADZENYS ER 4 AL1 Up to 25 yrs old QL 30 / 30 Days ADZENYS XR-ODT 4 AL1 Up to 25 yrs old QL 300 / 30 day(s) AMPHETAMINE ER 4 AL1 Up to 25 yrs old QL 180 / 30 day(s) amphetamine -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

United States Patent (19) 11 Patent Number: 4,765,973 Heller 45 Date of Patent: Aug
United States Patent (19) 11 Patent Number: 4,765,973 Heller 45 Date of Patent: Aug. 23, 1988 54) POLYMERS CONTAINING PEN DANT ACD Assistant Examiner-M. L. Moore FUNCTIONALITIES AND LABILE Attorney, Agent, or Firm-Manfred Polk; Michael C. BACKBONE BONDS Sudol (75) Inventor: Jorge Heller, Woodside, Calif. 57 ABSTRACT Assignee: Merck & Co., Inc., Rahway, N.J. The instant invention is directed to a polymer with at 73 least one labile backbone bond per repeat unit and at (21) Appl. No.: 915,348 least one pendant acid functionality per thousand repeat 22 Filed: Oct. 6, 1986 units. The instant invention is also directed to a controlled Related U.S. Application Data release device which comprises: (A) a polymer with at least one labile backbone bond (62) Division of Ser. No. 68,002, Jun. 6, 1984, Pat. No. per repeat unit and at least one pendant acid func 4,639,366. tionality per thousand repeat units; and 51 Int. Cl'.............................................. A61K 31/74. (B) a beneficial substance incorporated within or 52) U.S. C. ....................................... 424/486; 424/78 surrounded by the matrix of said polymer. (58) Field of Search ........................ 424/19, 20, 22, 78 Primary Examiner-John Kight 3 Claims, No Drawings 4,765,973 1. 2 Tri- and higher hydroxyl functional polyols may be POLYMERS CONTAINING PEN DANT ACID used, which will result in crosslinked polymers. FUNCTIONALTES AND LABLE BACKBONE Any polymer containing at least one acid labile back BONDS bone bond per repeat unit (preferably two per repeat 5 acid unit) may be used to react with the polyol contain This is a division of application Ser. -

Demecarium Bromide/Homatropine 1881
Demecarium Bromide/Homatropine 1881 Dyflos (BAN) junctival injection of pralidoxime has been used to reverse severe ocular adverse effects. Supportive treatment, including assisted DFP; Difluorophate; Di-isopropyl Fluorophosphate; Di-isopro- ventilation, should be given as necessary. CH3 pylfluorophosphonate; Fluostigmine; Isoflurofato; Isoflurophate. Di-isopropyl phosphorofluoridate. To prevent or reduce development of iris cysts in patients receiv- N ing ecothiopate eye drops, phenylephrine eye drops may be giv- C6H14FO3P = 184.1. en simultaneously. CAS — 55-91-4. ATC — S01EB07. Precautions ATC Vet — QS01EB07. As for Neostigmine, p.632. For precautions of miotics, see also OH under Pilocarpine, p.1885. In general, as with other long-acting anticholinesterases, ecothiopate should be used only where ther- O apy with other drugs has proved ineffective. Ecothiopate iodide CH3 should not be used in patients with iodine hypersensitivity. O O H3C O Interactions P CH3 As for Neostigmine, p.632. The possibility of an interaction re- O F mains for a considerable time after stopping long-acting anti- Homatropine Hydrobromide (BANM) CH3 cholinesterases such as ecothiopate. Homatr. Hydrobrom.; Homatropiinihydrobromidi; Homatropi- Pharmacopoeias. In US. Uses and Administration na, hidrobromuro de; Homatropine, bromhydrate d’; Homatro- USP 31 (Isoflurophate). A clear, colourless, or faintly yellow liq- Ecothiopate is an irreversible inhibitor of cholinesterase; its ac- pin-hidrobromid; Homatropinhydrobromid; Homatropin-hydro- uid. Specific gravity about 1.05. Sparingly soluble in water; sol- tions are similar to those of neostigmine (p.632) but much more bromid; Homatropini hydrobromidum; Homatropinium Bro- uble in alcohol and in vegetable oils. It is decomposed by mois- prolonged. Its miotic action begins within 1 hour of its applica- mide; Homatropino hidrobromidas; Homatropinum Bromatum; ture with the evolution of hydrogen fluoride. -

Malta Medicines List April 08
Defined Daily Doses Pharmacological Dispensing Active Ingredients Trade Name Dosage strength Dosage form ATC Code Comments (WHO) Classification Class Glucobay 50 50mg Alpha Glucosidase Inhibitor - Blood Acarbose Tablet 300mg A10BF01 PoM Glucose Lowering Glucobay 100 100mg Medicine Rantudil® Forte 60mg Capsule hard Anti-inflammatory and Acemetacine 0.12g anti rheumatic, non M01AB11 PoM steroidal Rantudil® Retard 90mg Slow release capsule Carbonic Anhydrase Inhibitor - Acetazolamide Diamox 250mg Tablet 750mg S01EC01 PoM Antiglaucoma Preparation Parasympatho- Powder and solvent for solution for mimetic - Acetylcholine Chloride Miovisin® 10mg/ml Refer to PIL S01EB09 PoM eye irrigation Antiglaucoma Preparation Acetylcysteine 200mg/ml Concentrate for solution for Acetylcysteine 200mg/ml Refer to PIL Antidote PoM Injection injection V03AB23 Zovirax™ Suspension 200mg/5ml Oral suspension Aciclovir Medovir 200 200mg Tablet Virucid 200 Zovirax® 200mg Dispersible film-coated tablets 4g Antiviral J05AB01 PoM Zovirax® 800mg Aciclovir Medovir 800 800mg Tablet Aciclovir Virucid 800 Virucid 400 400mg Tablet Aciclovir Merck 250mg Powder for solution for inj Immunovir® Zovirax® Cream PoM PoM Numark Cold Sore Cream 5% w/w (5g/100g)Cream Refer to PIL Antiviral D06BB03 Vitasorb Cold Sore OTC Cream Medovir PoM Neotigason® 10mg Acitretin Capsule 35mg Retinoid - Antipsoriatic D05BB02 PoM Neotigason® 25mg Acrivastine Benadryl® Allergy Relief 8mg Capsule 24mg Antihistamine R06AX18 OTC Carbomix 81.3%w/w Granules for oral suspension Antidiarrhoeal and Activated Charcoal