Organo Lithium Reagents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Direct Preparation of Some Organolithium Compounds from Lithium and RX Compounds Katashi Oita Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1955 Direct preparation of some organolithium compounds from lithium and RX compounds Katashi Oita Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Oita, Katashi, "Direct preparation of some organolithium compounds from lithium and RX compounds " (1955). Retrospective Theses and Dissertations. 14262. https://lib.dr.iastate.edu/rtd/14262 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overiaps. -

United States Patent (19) 11 Patent Number: 5,945,382 Cantegrill Et Al
US005.945382A United States Patent (19) 11 Patent Number: 5,945,382 Cantegrill et al. (45) Date of Patent: *Aug. 31, 1999 54 FUNGICIDAL ARYLPYRAZOLES 2300173 12/1990 Japan. 2224208 5/1990 United Kingdom. 75 Inventors: Richard Cantegril, Lyons; Denis Croisat, Paris; Philippe Desbordes, OTHER PUBLICATIONS Lyons, Francois Guigues, English translation of JP 2-300173, 1990. Rillieux-la-Pape; Jacques Mortier, La English translation of JP 59–53468, 1984. Bouéxier; Raymond Peignier, Caluire; English translation of JP 3-93774, 1991. Jean Pierre Vors, Lyons, all of France Miura et al., (CA 1.14:164226), 1991. Miura et al., (CA 115:92260), 1991. 73 Assignee: Rhone-Poulenc Agrochimie, Lyons, Chemical Abstracts, vol. 108, No. 23, 1986, abstract No. France 204577b. CAS Registry Handbook, No. section, RN=114913-44-9, * Notice: This patent is subject to a terminal dis 114486-01-0, 99067-15-9, 113140-19-5, 73227-97-1, claimer. 27069-17-6, 18099-21–3, 17978-27-7, 1988. 21 Appl. No.: 08/325,283 Hattori et al., CA 68:68981 (1968), Registry No. 17978–25–5, 17978-26-6, 17978-27-7 and 18099–21-3. 22 PCT Filed: Apr. 26, 1993 Hattori et al., CA 68:68982 (1968), Registry No. 17978-28-8. 86 PCT No.: PCT/FR93/00403 Janssen et al., CA 78: 159514 (1973), Registry No. S371 Date: Dec. 22, 1994 38858-97-8 and 38859-02-8. Chang et al., CA 92:146667 (1980), Registry No. S 102(e) Date: Dec. 22, 1994 73227 91-1. Berenyi et al., CA 94:156963 (1981), Registry No. -
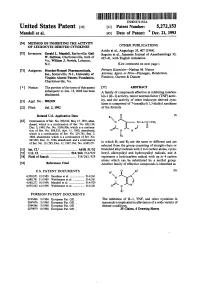
ESSEE in Which R1 and R2 Are the Same Or Different And
USOO5272153A United States Patent (19) 11 Patent Number: 5,272,153 Mandell et al. 45 Date of Patent: Dec. 21, 1993 (54)54 OFMETH E. NHIBITINSi6. CTVTY OTHER PUBLICATIONS Avido et al., Angiology 35, 407 (1984). 75 Inventors: St. Mel ES Suguira et al., Japanese Journal of Anesthesiology 32, va.8 William,Wan, CharlotteSVlie, Novici, Lebanon, O 435-41,35-41, with English translationtranslation. N.J. (List continued on next page.) 73) Assignees: Hoechst-Roussel Pharmaceuticals, Primary Examiner-Nathan M. Nutter Inc., Somerville, N.J.; University of Attorney, Agent, or Firm-Finnegan, Henderson, Virginia Alumni Patents Foundation, Farabow, Garrett & Dunner Charlottesville, Va. (*) Notice: The portion of the term of this patent 57) ABSTRACT E. to Jun 15, 2008 has been A family of compounds effective in inhibiting interleu SC. kin-1 (IL-1) activity, tumor necrosis factor (TNF) activ (21) Appl. No.: 908,929 ity, and the activity of other leukocyte derived cyto s kines is comprised of 7-(oxoalkyl) 1,3-dialkyl xanthines (22 Filed: Jul. 2, 1992 of the formula Related U.S. Application Data R (I) 63 Continuation of Ser. No. 700,522, May 15, 1991, aban- Y N-A-C-CH3 doned, which is a continuation of Ser. No. 622,138, Dec. 5, 1990, Pat. No. 5,096,906, which is a continua- al- 6 tion of Ser. No. 508,535, Apr. 11, 1990, abandoned, O N N which is a continuation of Ser. No. 239,761, Sep. 2, 1988, abandoned, which is a continuation of ser. No. R2 ESSEE selectedin which from R1 and the R2group are consistingthe same orof different straight-chain and are or 51) int. -

Lithium Isotope Effects Upon Electrochemical Release from Lithium Manganese Oxide
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 71 ( 2015 ) 140 – 148 The Fourth International Symposium on Innovative Nuclear Energy Systems, INES-4 Lithium isotope effects upon electrochemical release from lithium manganese oxide Koji OKANOa, Yuta TAKAMIa, Satoshi YANASEa, Takao OIa,* a Faculty of Science and Technology, Sophia University, 7-1 Kioicho, Chiyodaku, Tokyo, 102-8554 Japan Abstract Lithium was electrochemically released from lithium manganese oxide (LiMn2O4) to an electrolyte solution, a 1:2 v/v mixed solution of ethylene carbonate (EC) and ethylmethyl carbonate (EMC) containing 1 M lithium perchlorate or sodium perchlorate (EC/EMC/LiClO4 or EC/EMC/NaClO4) to observe the lithium isotope effects that accompanied the lithium release. The lighter isotope of lithium, 6Li, was preferentially fractionated to the electrolyte solution phase, with the value of the lithium isotope separation factor ranging from 0.989 to 0.971 at 25 ºC. The degree of the lithium isotope fractionation was slightly smaller in the LiMn2O4-EC/EMC/NaClO4 system than in the LiMn2O4-EC/EMC/LiClO4 system. The present systems are in great contrast with the lithium cobalt oxide (LiCoO2)-the electrolyte solution systems concerning the direction and magnitude of the lithium isotope effects, which seems mostly ascribable to the structural difference between LiMn2O4 and LiCoO2ࠋ © 20152014 TheThe Authors. Authors. Published Published by Elsevierby Elsevier Ltd. Ltd.This is an open access article under the CC BY-NC-ND license Selection(http://creativecommons.org/licenses/by-nc-nd/3.0/ and peer-review under responsibility). of the Tokyo Institute of Technology. Selection and peer-review under responsibility of the Tokyo Institute of Technology Keywords: lithium isotopes; isotope effects; lithium manganese oxide; separation factor; lithium ion secondary batteries; ethylene carbonate 1. -

A Study of Lithium Precursors on Nanoparticle Quality
Electronic Supplementary Material (ESI) for Nanoscale. This journal is © The Royal Society of Chemistry 2021 Electronic Supplementary Information Elucidating the role of precursors in synthesizing single crystalline lithium niobate nanomaterials: A study of lithium precursors on nanoparticle quality Rana Faryad Ali, Byron D. Gates* Department of Chemistry and 4D LABS, Simon Fraser University, 8888 University Drive Burnaby, BC, V5A 1S6, Canada * E-mail: [email protected] This work was supported in part by the Natural Sciences and Engineering Research Council of Canada (NSERC; Grant No. RGPIN-2020-06522), and through the Collaborative Health Research Projects (CHRP) Partnership Program supported in part by the Canadian Institutes of Health Research (Grant No. 134742) and the Natural Science Engineering Research Council of Canada (Grant No. CHRP 462260), the Canada Research Chairs Program (B.D. Gates, Grant No. 950-215846), CMC Microsystems (MNT Grant No. 6345), and a Graduate Fellowship (Rana Faryad Ali) from Simon Fraser University. This work made use of 4D LABS (www.4dlabs.com) and the Center for Soft Materials shared facilities supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF), Western Economic Diversification Canada, and Simon Fraser University. S1 Experimental Materials and supplies All chemicals were of analytical grade and were used as received without further purification. Niobium ethoxide [Nb(OC2H5)5, >90%] was obtained from Gelest Inc., and benzyl alcohol (C7H7OH, 99%) and triethylamine [N(C2H5)3, 99.0%] were purchased from Acros Organics and Anachemia, respectively. Lithium chloride (LiCl, ~99.0%) was obtained from BDH Chemicals, and lithium bromide (LiBr, ≥99.0%), lithium fluoride (LiF, ~99.9%), and lithium iodide (LiI, 99.0%) were purchased from Sigma Aldrich. -

OMLI041 GHS US English US
OMLI041 - METHYLLITHIUM, 3M in diethoxymethane METHYLLITHIUM, 3M in diethoxymethane Safety Data Sheet OMLI041 Date of issue: 10/04/2017 Revision date: 05/24/2018 Version: 1.1 SECTION 1: Identification 1.1. Identification Product name : METHYLLITHIUM, 3M in diethoxymethane Product code : OMLI041 Product form : Mixture Physical state : Liquid Formula : CH3Li Synonyms : LITHIUM METHIDE Chemical family : METAL ALKYL 1.2. Recommended use and restrictions on use Recommended use : Chemical intermediate 1.3. Supplier GELEST, INC. 11 East Steel Road Morrisville, PA 19067 USA T 215-547-1015 - F 215-547-2484 - (M-F): 8:00 AM - 5:30 PM EST [email protected] - www.gelest.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 (USA); +1 703-527-3887 (International) SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Flammable liquids Category 2 H225 Highly flammable liquid and vapor Pyrophoric liquids Category 1 H250 Catches fire spontaneously if exposed to air Substances and mixtures which in contact with water emit flammable H260 In contact with water releases flammable gases which may ignite gases Category 1 spontaneously Skin corrosion/irritation Category 1B H314 Causes severe skin burns and eye damage Serious eye damage/eye irritation Category 1 H318 Causes serious eye damage Specific target organ toxicity (single exposure) Category 3 H335 May cause respiratory irritation Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS US labeling Hazard pictograms (GHS US) : Signal word (GHS US) : Danger Hazard statements (GHS US) : H225 - Highly flammable liquid and vapor H250 - Catches fire spontaneously if exposed to air H260 - In contact with water releases flammable gases which may ignite spontaneously H314 - Causes severe skin burns and eye damage H318 - Causes serious eye damage H335 - May cause respiratory irritation Precautionary statements (GHS US) : P280 - Wear protective gloves/protective clothing/eye protection/face protection. -

Synthesis, Reactivity, and Catalysis of Group 3 and Lanthanide Alkyl Complexes
Synthesis, Reactivity, and Catalysis of Group 3 and Lanthanide Alkyl Complexes By Daniel Steven Levine A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry in the Graduate Division of the University of California, Berkeley Committee in charge: Professor T. Don Tilley, Co-Chair Professor Richard A. Andersen, Co-Chair Professor Alexis T. Bell Summer 2016 Abstract Synthesis, Reactivity, and Catalysis of Group 3 and Lanthanide Alkyl Complexes by Daniel Steven Levine Doctor of Philosophy in Chemistry University of California, Berkeley Professor T. Don Tilley, Co-Chair Professor Richard A. Andersen, Co-Chair Chapter 1. A series of scandium dialkyl complexes, (PNP)ScR2 (R = neopentyl, trimethylsilylmethyl), supported by the monoanionic, chelating PNP ligand (2,5- bis(dialkylphosphinomethyl)pyrrolide; alkyl = cyclohexyl, tert-butyl) was synthesized and the reactivities of these complexes toward simple hydrocarbons was investigated. The scandium– carbon bonds undergo σ-bond metathesis reactions with hydrogen and these complexes are catalysts for the hydrogenation of alkenes. Reactions with primary amines led to formation of amido complexes that undergo cyclometalation via σ-bond metathesis, without involvement of an imido complex intermediate. A variety of carbon-hydrogen bonds are also activated, including sp-, sp2-, and sp3-C–H bonds (intramolecularly in the latter case). Levine, D. S.; Tilley, T. D.; Andersen, R. A. Organometallics 2015, 34 (19), 4647. Chapter 2. Terminal group 3 methylidene complexes are generated by thermolysis of monoanionic PNP-supported scandium and yttrium dialkyl complexes. The reaction mechanism has been probed by deuterium-labeling experiments and DFT calculations. Abstraction of a γ- hydrogen from one alkyl group by the other affords a metallacyclobutane that undergoes [2+2] cycloreversion, analogous to a key step in the olefin metathesis reaction, to generate a methylidene complex and isobutene. -

Organic Seminar Abstracts
L I B RA FLY OF THE U N IVERSITY OF 1LLI NOIS Q.54-1 Ii£s 1964/65 ,t.l P Return this book on or before the Latest Date stamped below. Theft, mutilation, and underlining of books are reasons for disciplinary action and may result in dismissal from the University. University of Illinois Library OCfcfcsrm L161— O-1096 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/organicsemi1964651univ s ORGANIC SEMINAR ABSTRACTS 196^-65 Semester I Department of Chemistry and Chemical Engineering University of Illinois ' / SEMINAR TOPICS I Semester I96U-I965 f"/ ( Orientation in Sodium and Potassium Metalations of Aromatic Compounds Earl G. Alley 1 Structure of Cyclopropane Virgil Weiss 9 Vinylidenes and Vinylidenecarbenes Joseph C. Catlin 17 Diazene Intermediates James A. Bonham 25 Perfluoroalkyl and Polyfluoroalkyl Carbanions J. David Angerer 3^ Free Radical Additions to Allenes Raymond Feldt 43 The Structure and Biosynthesis of Quassin Richard A. Larson 52 The Decomposition of Perester Compounds Thomas Sharpe 61 The Reaction of Di-t -Butyl Peroxide with Simple Alkyl, Benzyl and Cyclic Ethers R. L. Keener 70 Rearrangements and Solvolysis in Some Allylic Systems Jack Timberlake 79 Longifolene QQ Michael A. Lintner 00 Hydrogenation ! Homogeneous Catalytic Robert Y. Ning 9° c c Total Synthesis of ( -) -Emetime R. Lambert 1* Paracyclophanes Ping C. Huang 112 Mechanism of the Thermal Rearrangement of Cyclopropane George Su 121 Some Recent Studies of the Photochemistry of Cross -Conjugated Cyclohexadienones Elizabeth McLeister 130 The Hammett Acidity Function R. P. Quirk 139 Homoaromaticity Roger A. -

Silyl Ketone Chemistry. Preparation and Reactions of Silyl Allenol Ethers. Diels-Alder Reactions of Siloxy Vinylallenes Leading to Sesquiterpenes2
J. Am. Chem. SOC.1986, 108, 7791-7800 7791 pyrany1oxy)dodecanoic acid, 1.38 1 g (3.15 mmol) of GPC-CdCIz, 0.854 product mixture was then filtered and concentrated under reduced g (7.0 mmol) of 4-(dimethylamino)pyridine, and 1.648 g (8.0 mmol) of pressure. The residue was dissolved in 5 mL of solvent B and passed dicyclohexylcarbodiimide was suspended in 15 mL of dry dichloro- through a 1.2 X 1.5 cm AG MP-50 cation-exchange column in order to methane and stirred under nitrogen in the dark for 40 h. After removal remove 4-(dimethylamino)pyridine. The filtrate was concentrated under of solvent in vacuo, the residue was dissolved in 50 mL of CH30H/H20 reduced pressure, dissolved in a minimum volume of absolute ethanol, (95/5, v/v) and stirred in the presence of 8.0 g of AG MP-50 (23 OC, and then concentrated again. Chromatographic purification of the res- 2 h) to allow for complete deprotection of the hydroxyl groups (monitored idue on a silica gel column (0.9 X 6 cm), eluting first with solvent A and by thin-layer chromatography)." The resin was then removed by fil- then with solvent C (compound 1 elutes on silica as a single yellow band), tration and the solution concentrated under reduced pressure. The crude afforded, after drying [IO h, 22 OC (0.05 mm)], 0.055 g (90%) of 1 as product (2.75 g). obtained after drying [12 h, 23 OC (0.05 mm)], was a yellow solid: R 0.45 (solvent C); IR (KBr) ucz0 1732, uN(cH3)3 970, then subjected to chromatographic purification by using a 30-g (4 X 4 1050, 1090cm-'; I' H NMR (CDCI,) 6 1.25 (s 28 H, CH2), 1.40-2.05 (m, cm) silica gel column, eluting with solvents A and C, to yield 0.990 g 20 H, lipoic-CH,, CH2CH20,CH2CH,C02), 2.3 (t. -

Polyorganosiloxanes: Molecular Nanoparticles, Nanocomposites and Interfaces
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses November 2017 POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES Daniel H. Flagg University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Materials Chemistry Commons, Polymer and Organic Materials Commons, and the Polymer Chemistry Commons Recommended Citation Flagg, Daniel H., "POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES" (2017). Doctoral Dissertations. 1080. https://doi.org/10.7275/10575940.0 https://scholarworks.umass.edu/dissertations_2/1080 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the degree requirements for the degree of DOCTOR OF PHILOSOPHY September 2017 Polymer Science and Engineering © Copyright by Daniel H. Flagg 2017 All Rights Reserved POLYORGANOSILOXANES: MOLECULAR NANOPARTICLES, NANOCOMPOSITES AND INTERFACES A Dissertation Presented by Daniel H. Flagg Approved as to style and content by: Thomas J. McCarthy, Chair E. Bryan Coughlin, Member John Klier, Member E. Bryan Coughlin, Head, PS&E To John Null ACKNOWLEDGEMENTS There are countless individuals that I need to thank and acknowledge for getting me to where I am today. I could not have done it alone and would be a much different person if it were not for the support of my advisors, friends and family. -

(12) United States Patent (10) Patent No.: US 9.209,487 B2 Scanlon, Jr
US0092094.87B2 (12) United States Patent (10) Patent No.: US 9.209,487 B2 Scanlon, Jr. et al. (45) Date of Patent: Dec. 8, 2015 (54) SOLD-STATE ELECTROLYTES FOR HO1 M 4/134 (2010.01) RECHARGEABLE LITHIUM BATTERIES HOIM 4/40 (2006.01) (52) U.S. C. (71) Applicant: The United States of America, as CPC ............ H0IM 10/0564 (2013.01); H01 M 4/60 Represented by the Secretary of the (2013.01); H0 IM 4/62 (2013.01); H0IM Air Force, Washington, DC (US) 10/0525 (2013.01); H01 M 4/134 (2013.01); H01 M 4/405 (2013.01); HOIM 2300/0065 (72) Inventors: Lawrence G Scanlon, Jr., Fairborn, OH (2013.01); HOIM 2300/0091 (2013.01); Y02E (US); Joseph P Fellner, Kettering, OH 60/122 (2013.01) (US); William A. Feld, Beavercreek, OH (58) Field of Classification Search (US); Leah R. Lucente, Beavercreek, None OH (US); Jacob W. Lawson, See application file for complete search history. Springfield, OH (US); Andrew M. Beauchamp, Lancaster, CA (US) (56) References Cited U.S. PATENT DOCUMENTS (73) Assignee: The United States of America as represented by the Secretary of the Air 5,932,133 A 8/1999 Scanlon, Jr. Force, Washington, DC (US) 6,010,805 A 1/2000 Scanlon, Jr. et al. 6,541,161 B1 4/2003 Scanlon, Jr. (*) Notice: Subject to any disclaimer, the term of this 2013/0309561 A1 11/2013 Chen et al. patent is extended or adjusted under 35 (Continued) U.S.C. 154(b) by 0 days. OTHER PUBLICATIONS (21) Appl. No.: 14/333,836 L. -
Oxygen Reduction Via Iodide Redox Mediation in Li-O2 Batteries
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/308004421 Implications of 4 e- Oxygen Reduction via Iodide Redox Mediation in Li-O2 Batteries Article in ACS Energy Letters · September 2016 DOI: 10.1021/acsenergylett.6b00328 CITATIONS READS 3 206 7 authors, including: Vincent Giordani Dan Addison Liox Power, Inc. Independent Researcher 30 PUBLICATIONS 769 CITATIONS 30 PUBLICATIONS 400 CITATIONS SEE PROFILE SEE PROFILE Linda F. Nazar Bryan Mccloskey University of Waterloo University of California, Berkeley 264 PUBLICATIONS 19,366 CITATIONS 66 PUBLICATIONS 4,941 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Vincent Giordani on 15 September 2016. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. Letter http://pubs.acs.org/journal/aelccp Implications of 4 e− Oxygen Reduction via − Iodide Redox Mediation in Li O2 Batteries Colin M. Burke,†,‡,# Robert Black,§,∥,# Ivan R. Kochetkov,§,∥ Vincent Giordani,⊥ Dan Addison,*,⊥ Linda F. Nazar,*,§,∥ and Bryan D. McCloskey*,†,‡ † Department of Chemical and Biomolecular Engineering, University of California, Berkeley, California 94720, United States ‡ Energy Storage and Distributed Resources Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States § University of Waterloo, Department of Chemistry, Waterloo, Ontario N2L 3G1, Canada ∥ Waterloo Institute for Nanotechnology, Waterloo, Ontario N2L 3G1, Canada ⊥ Liox Power Inc., 129 North Hill Avenue, Pasadena, California 91106, United States *S Supporting Information − − ABSTRACT: The nonaqueous lithium oxygen (Li O2) electrochemistry has garnered significant attention because of its high theoretical specific energy compared to the state-of-the-art lithium-ion battery.