Wurmbea Latifolia Subsp. Vanessae R.J.Bates, J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nuytsia the Journal of the Western Australian Herbarium 31: 197–202 Published Online 20 August 2020
T.D. Macfarlane, A.P. Brown & C.J. French, Wurmbea flavanthera (Colchicaceae), a new species 197 Nuytsia The journal of the Western Australian Herbarium 31: 197–202 Published online 20 August 2020 Wurmbea flavanthera (Yellow-anthered Wurmbea, Colchicaceae), a new species from Western Australia’s Mid West region Terry D. Macfarlane1, Andrew P. Brown and Christopher J. French Western Australian Herbarium, Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre, Western Australia 6983 1Corresponding author, email: [email protected] SHORT COMMUNICATION Wurmbea densiflora (Benth.) T.Macfarlane (Colchicaceae) is an attractive, small geophyte bearing several pink flowers, occurring in the northern Wheatbelt and adjacent rangelands of Western Australia. However, the species concept used in a revision of Wurmbea Thunb. in Australia (Macfarlane 1980, 1987) proves to be a mixture of two species, W. densiflora in the original sense of Bentham (1878) and a later-flowering, undescribed species. This second species was given the phrase nameW . sp. Paynes Find (C.J. French 1237), by which it has been known for some time (Western Australian Herbarium 1998–). The new species, having been well-researched in the field and Herbarium, is formally described below. The previous confusion between the two species was partly due to a lack of field knowledge and an inadequate appreciation of the relatively constrained flowering times ofWurmbea species. Wurmbea flavanthera T.Macfarlane, A.P.Br. & C.J.French, sp. nov. Type: 11.8 km north of Jose Street, Mullewa, on Carnarvon – Mullewa Road, west of road on a minor track, Western Australia, 17 August 2011, T.D. -

Partial Flora Survey Rottnest Island Golf Course
PARTIAL FLORA SURVEY ROTTNEST ISLAND GOLF COURSE Prepared by Marion Timms Commencing 1 st Fairway travelling to 2 nd – 11 th left hand side Family Botanical Name Common Name Mimosaceae Acacia rostellifera Summer scented wattle Dasypogonaceae Acanthocarpus preissii Prickle lily Apocynaceae Alyxia Buxifolia Dysentry bush Casuarinacea Casuarina obesa Swamp sheoak Cupressaceae Callitris preissii Rottnest Is. Pine Chenopodiaceae Halosarcia indica supsp. Bidens Chenopodiaceae Sarcocornia blackiana Samphire Chenopodiaceae Threlkeldia diffusa Coast bonefruit Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Suada australis Seablite Chenopodiaceae Atriplex isatidea Coast saltbush Poaceae Sporabolis virginicus Marine couch Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Pittosporaceae Pittosporum phylliraeoides Weeping pittosporum Poaceae Stipa flavescens Tussock grass 2nd – 11 th Fairway Family Botanical Name Common Name Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Atriplex isatidea Coast saltbush Cyperaceae Gahnia trifida Coast sword sedge Pittosporaceae Pittosporum phyliraeoides Weeping pittosporum Myrtaceae Melaleuca lanceolata Rottnest Is. Teatree Chenopodiaceae Sarcocornia blackiana Samphire Central drainage wetland commencing at Vietnam sign Family Botanical Name Common Name Chenopodiaceae Halosarcia halecnomoides Chenopodiaceae Sarcocornia quinqueflora Beaded samphire Chenopodiaceae Sarcocornia blackiana Samphire Poaceae Sporobolis virginicus Cyperaceae Gahnia Trifida Coast sword sedge -

Tracing History
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 911 Tracing History Phylogenetic, Taxonomic, and Biogeographic Research in the Colchicum Family BY ANNIKA VINNERSTEN ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2003 Dissertation presented at Uppsala University to be publicly examined in Lindahlsalen, EBC, Uppsala, Friday, December 12, 2003 at 10:00 for the degree of Doctor of Philosophy. The examination will be conducted in English. Abstract Vinnersten, A. 2003. Tracing History. Phylogenetic, Taxonomic and Biogeographic Research in the Colchicum Family. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 911. 33 pp. Uppsala. ISBN 91-554-5814-9 This thesis concerns the history and the intrafamilial delimitations of the plant family Colchicaceae. A phylogeny of 73 taxa representing all genera of Colchicaceae, except the monotypic Kuntheria, is presented. The molecular analysis based on three plastid regions—the rps16 intron, the atpB- rbcL intergenic spacer, and the trnL-F region—reveal the intrafamilial classification to be in need of revision. The two tribes Iphigenieae and Uvularieae are demonstrated to be paraphyletic. The well-known genus Colchicum is shown to be nested within Androcymbium, Onixotis constitutes a grade between Neodregea and Wurmbea, and Gloriosa is intermixed with species of Littonia. Two new tribes are described, Burchardieae and Tripladenieae, and the two tribes Colchiceae and Uvularieae are emended, leaving four tribes in the family. At generic level new combinations are made in Wurmbea and Gloriosa in order to render them monophyletic. The genus Androcymbium is paraphyletic in relation to Colchicum and the latter genus is therefore expanded. -
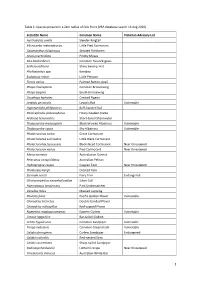
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Ecological Differentiation of Combined and Separate Sexes of Wurmbea Dioica (Colchicaceae) in Sympatry
Ecology, 82(9), 2001, pp. 2601±2616 q 2001 by the Ecological Society of America ECOLOGICAL DIFFERENTIATION OF COMBINED AND SEPARATE SEXES OF WURMBEA DIOICA (COLCHICACEAE) IN SYMPATRY ANDREA L. CASE1 AND SPENCER C. H. BARRETT Department of Botany, University of Toronto, 25 Willcocks Street, Toronto, Ontario, Canada M5S 3B2 Abstract. The evolution and maintenance of combined vs. separate sexes in ¯owering plants is in¯uenced by both ecological and genetic factors; variation in resources, partic- ularly moisture availability, is thought to play a role in selection for gender dimorphism in some groups. We investigated the density, distribution, biomass allocation, and physi- ology of sympatric monomorphic (cosexual) and dimorphic (female and male) populations of Wurmbea dioica in relation to soil moisture on the Darling Escarpment in southwestern Australia. Populations with monomorphic vs. dimorphic sexual systems segregated into wet vs. dry microsites, respectively, and biomass allocation patterns and physiological traits re¯ected differences in water availability, despite similarities in total ramet biomass between the sexual systems. Unisexuals ¯owered earlier at lower density, and they allocated sig- ni®cantly more biomass below ground to roots and corms than did cosexuals, which al- located more biomass above ground to leaves, stems, and ¯owers. Females, males, and cosexuals produced similar numbers of ¯owers per ramet, but unisexuals produced more ramets than cosexuals, increasing the total number of ¯owers per genet. Contrary to ex- pectation, cosexuals had signi®cantly higher (more positive) leaf carbon isotope ratios and lower leaf nitrogen content than unisexuals, suggesting that cosexuals are more water-use ef®cient and have lower rates of photosynthesis per unit leaf mass despite their occurrence in wetter microsites. -

The Species of Wurmbea
J. Adelaide Bot. Gard. 16: 33-53 (1995) THE SPECIES OFWURMBEA(LILIACEAE) IN SOUTH AUSTRALIA Robert J. Bates Cl- State Herbarium, Botanic Gardens, North Terrace, Adelaide, South Australia 5000 Abstract Nine species of Wunnbea Thunb. are recognised in South Australia. W. biglandulosa (R. Br.)Macfarlane, W. deserticola Macfarlane and W. sinora Macfarlane are recorded for the first time; Wurmbea biglandulosa ssp. flindersica, W. centralis ssp. australis, W. decumbens, W. dioica ssp. citrina, W. dioica ssp. lacunaria, W. latifolia ssp. vanessae and W. stellata are described. A key, together with notes on each species is provided. Macfarlane (1980) revised the genus for Australia. He placed Anguillaria R. Br. under Wurmbea and recognised W. dioica (R. Br.)F. Muell., W. centralis Macfarlane, W. latifolia Macfarlane and W. uniflora (R. Br.)Macfarlane as occurring in South Australia. Before this only one species, W. dioica (as Anguillaria dioica) was listed for South Australia (J.M. Black 1922, 1943). Macfarlane stated that he had seen no live material of South Australian species. The present author has made extensive field studies of taxa discussed in this paper, has cultivated most and studied herbarium material. Several trips have been made to other states to allow further comparisons to be made. For information on the nomenclatural history, general morphology, biology and ecology of Wurmbea see Macfarlane 1980. Key to the South Australian species of Wurmbea 1 Lower leaves paired (almost opposite), basal, of same shape and size 2 1: Lower leaves well separated, often of different shape and size 4 2 Leaves with serrate margins, flowers unisexual, nectaries 1 per tepal, a single band of colour... -

Albuca Spiralis
Flowering Plants of Africa A magazine containing colour plates with descriptions of flowering plants of Africa and neighbouring islands Edited by G. Germishuizen with assistance of E. du Plessis and G.S. Condy Volume 62 Pretoria 2011 Editorial Board A. Nicholas University of KwaZulu-Natal, Durban, RSA D.A. Snijman South African National Biodiversity Institute, Cape Town, RSA Referees and other co-workers on this volume H.J. Beentje, Royal Botanic Gardens, Kew, UK D. Bridson, Royal Botanic Gardens, Kew, UK P. Burgoyne, South African National Biodiversity Institute, Pretoria, RSA J.E. Burrows, Buffelskloof Nature Reserve & Herbarium, Lydenburg, RSA C.L. Craib, Bryanston, RSA G.D. Duncan, South African National Biodiversity Institute, Cape Town, RSA E. Figueiredo, Department of Plant Science, University of Pretoria, Pretoria, RSA H.F. Glen, South African National Biodiversity Institute, Durban, RSA P. Goldblatt, Missouri Botanical Garden, St Louis, Missouri, USA G. Goodman-Cron, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, RSA D.J. Goyder, Royal Botanic Gardens, Kew, UK A. Grobler, South African National Biodiversity Institute, Pretoria, RSA R.R. Klopper, South African National Biodiversity Institute, Pretoria, RSA J. Lavranos, Loulé, Portugal S. Liede-Schumann, Department of Plant Systematics, University of Bayreuth, Bayreuth, Germany J.C. Manning, South African National Biodiversity Institute, Cape Town, RSA A. Nicholas, University of KwaZulu-Natal, Durban, RSA R.B. Nordenstam, Swedish Museum of Natural History, Stockholm, Sweden B.D. Schrire, Royal Botanic Gardens, Kew, UK P. Silveira, University of Aveiro, Aveiro, Portugal H. Steyn, South African National Biodiversity Institute, Pretoria, RSA P. Tilney, University of Johannesburg, Johannesburg, RSA E.J. -

Flora Surveys Introduction Survey Method Results
Hamish Saunders Memorial Island Survey Program 2009 45 Flora Surveys The most studied island is Sarah Results Island. This island has had several Introduction plans developed that have A total of 122 vascular flora included flora surveys but have species from 56 families were There have been few flora focused on the historical value of recorded across the islands surveys undertaken in the the island. The NVA holds some surveyed. The species are Macquarie Harbour area. Data on observations but the species list comprised of 50 higher plants the Natural Values Atlas (NVA) is not as comprehensive as that (7 monocots and 44 dicots) shows that observations for given in the plans. The Sarah and 13 lower plants. Of the this area are sourced from the Island Visitor Services Site Plan species recorded 14 are endemic Herbarium, projects undertaken (2006) cites a survey undertaken to Australia; 1 occurs only in by DPIPWE (or its predecessors) by Walsh (1992). The species Tasmania. Eighteen species are such as the Huon Pine Survey recorded for Sarah Island have considered to be primitive. There and the Millennium Seed Bank been added to some of the tables were 24 introduced species found Collection project. Other data in this report. with 9 of these being listed weeds. has been added to the NVA as One orchid species was found part of composite data sets such Survey Method that was not known to occur in as Tasforhab and wetforest data the south west of the state and the sources of which are not Botanical surveys were this discovery has considerably easily traceable. -

The Vegetation Communities Native Grassland
Edition 2 From Forest to Fjaeldmark The Vegetation Communities Native grassland Themeda australis Edition 2 From Forest to Fjaeldmark (revised - October 2017) 1 Native grassland Community (Code) Page Coastal grass and herbfield (GHC) 5 Highland Poa grassland (GPH) 7 Lowland grassland complex (GCL) 9 Lowland grassy sedgeland (GSL) 11 Lowland Poa labillardierei grassland (GPL) 12 Lowland Themeda triandra grassland (GTL) 14 Rockplate grassland (GRP) 16 General description align the key and the description of Lowland grassland complex ( ) with respect to the Native grasslands are defined as areas of native GCL required cover of native grass species. In 2017 vegetation dominated by native grasses with few or further minor revisions were made to improve no emergent woody species. Different types of information for general management issues, and to native grassland can be found in a variety of habitats, improve the description of Coastal grass and including coastal fore-dunes, dry slopes and valley herbfield (GHC) and its differentiation from Lowland bottoms, rock plates and subalpine flats. The lowland Poa labillardierei grassland (GPL). This is reflected in temperate grassland types have been recognised as the key to this Section. some of the most threatened vegetation communities in Australia. General management issues Some areas of native grassland are human-induced Most lowland native grassland in Tasmania has been and exist as a result of heavy burning, tree clearing cleared for agriculture since European settlement or dieback of the tree layer in grassy woodlands. (Barker 1999, Gilfedder 1990, Kirkpatrick et al. 1988, There are seven grassland communities recognised Kirkpatrick 1991, Williams et al. 2007). -
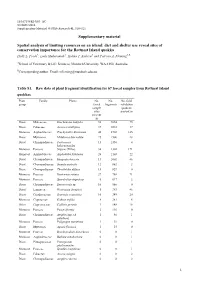
Supplementary Material Spatial Analysis of Limiting Resources on An
10.1071/WR14083_AC ©CSIRO 2014 Supplementary Material: Wildlife Research 41 , 510–521 Supplementary material Spatial analysis of limiting resources on an island: diet and shelter use reveal sites of conservation importance for the Rottnest Island quokka Holly L. Poole A, Laily Mukaromah A, Halina T. Kobryn A and Patricia A. Fleming A,B ASchool of Veterinary & Life Sciences, Murdoch University, WA 6150, Australia. BCorresponding author. Email: [email protected] Table S1. Raw data of plant fragment identification for 67 faecal samples from Rottnest Island quokkas Plant Family Plants No. No. No. field group faecal fragments validation sample quadrats sites present in present in Dicot Malvaceae Guichenotia ledifolia 52 9854 75 Dicot Fabaceae Acacia rostellifera 37 3018 37 Monocot Asphodelaceae Trachyandra divaricata 46 2702 145 Dicot Myrtaceae Melaleuca lanceolata 25 1506 28 Dicot Chenopodiaceae Tecticornia 13 1350 4 halocnemoides Monocot Poaceae Stipeae (Tribe) 34 1302 171 Monocot Asphodelaceae Asphodelus fistulosus 26 1103 22 Dicot Chenopodiaceae Rhagodia baccata 13 1002 46 Dicot Chenopodiaceae Suaeda australis 12 862 2 Dicot Chenopodiaceae Threlkeldia diffusa 15 829 0 Monocot Poaceae Rostraria cristata 27 788 71 Monocot Poaceae Sporobolus virginicus 5 617 2 Dicot Chenopodiaceae Sarcocornia sp . 10 560 0 Dicot Lamiaceae Westringia dampieri 5 383 46 Dicot Goodeniaceae Scaevola crassifolia 10 349 20 Monocot Cyperaceae Gahnia trifida 8 281 6 Other Cupressaceae Callitris preissii 3 148 18 Monocot Poaceae Poa poiformis 2 116 0 Dicot Chenopodiaceae Atriplex spp. (A. 1 40 1 paludosa ) Monocot Poaceae Polypogon maritimus 1 39 0 Dicot Myrtaceae Agonis flexuosa 1 15 0 Monocot Poaceae Brachypodium distachyon 0 0 1 Monocot Asphodelaceae Bulbine semibarbata 0 0 1 Dicot Pittosporaceae Pittosporum 0 0 1 phylliraeoides Monocot Poaceae Spinifex longifolius 0 0 1 Dicot Fabaceae Acacia saligna 0 0 2 Dicot Chenopodiaceae Atriplex cinerea 0 0 2 1 Dicot Asteraceae Centaurea sp . -

Gardens and Stewardship
GARDENS AND STEWARDSHIP Thaddeus Zagorski (Bachelor of Theology; Diploma of Education; Certificate 111 in Amenity Horticulture; Graduate Diploma in Environmental Studies with Honours) Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy October 2007 School of Geography and Environmental Studies University of Tasmania STATEMENT OF AUTHENTICITY This thesis contains no material which has been accepted for any other degree or graduate diploma by the University of Tasmania or in any other tertiary institution and, to the best of my knowledge and belief, this thesis contains no copy or paraphrase of material previously published or written by other persons, except where due acknowledgement is made in the text of the thesis or in footnotes. Thaddeus Zagorski University of Tasmania Date: This thesis may be made available for loan or limited copying in accordance with the Australian Copyright Act of 1968. Thaddeus Zagorski University of Tasmania Date: ACKNOWLEDGEMENTS This thesis is not merely the achievement of a personal goal, but a culmination of a journey that started many, many years ago. As culmination it is also an impetus to continue to that journey. In achieving this personal goal many people, supervisors, friends, family and University colleagues have been instrumental in contributing to the final product. The initial motivation and inspiration for me to start this study was given by Professor Jamie Kirkpatrick, Dr. Elaine Stratford, and my friend Alison Howman. For that challenge I thank you. I am deeply indebted to my three supervisors Professor Jamie Kirkpatrick, Dr. Elaine Stratford and Dr. Aidan Davison. Each in their individual, concerted and special way guided me to this omega point.