214Casonato #248.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Species of the Box-Gum Woodlands and Derived Native Grasslands
White Box-Yellow Box-Blakely’s Red Gum Grassy Woodland and Derived Native Grassland Ecological Community Species List White Box-Yellow Box-Blakely’s Red Gum Grassy Woodland and Derived Native Grassland Ecological Community Species List This species list is designed to provide information about plant species that can be found in the White Box-Yellow Box-Blakely’s Red Gum Grassy Woodland and Derived Native Grassland ecological community listed under the Environment Protection and Biodiversity Conservation Act 1999. The species list was developed to complement the Listing Information Guide, and should be read in that context. It provides information on scientific and common names of the species, the kind of plant the species is, whether it is an ‘important’ species for the purposes of this ecological community and whether it is exotic or native, perennial or annual. The list is not exhaustive and not all of the species listed will occur in every patch of White Box-Yellow Box-Blakely’s Red Gum Grassy Woodland and Derived Native Grassland. If there are any species that you think should be added to the list, removed from the list, or that are categorised incorrectly, please contact [email protected]. As such, this document may change over time and you should check that you are referring to the most recent version of the list. Caveat: This list has been compiled from a range of sources. While reasonable efforts have been made to ensure the accuracy of the information, no guarantee is given, nor responsibility taken, by the Commonwealth for its accuracy, currency or completeness. -
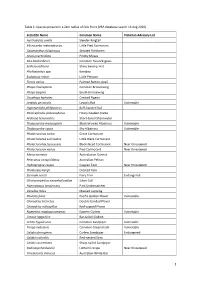
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Introduction Methods Results
Papers and Proceedings Royal Society ofTasmania, Volume 1999 103 THE CHARACTERISTICS AND MANAGEMENT PROBLEMS OF THE VEGETATION AND FLORA OF THE HUNTINGFIELD AREA, SOUTHERN TASMANIA by J.B. Kirkpatrick (with two tables, four text-figures and one appendix) KIRKPATRICK, J.B., 1999 (31:x): The characteristics and management problems of the vegetation and flora of the Huntingfield area, southern Tasmania. Pap. Proc. R. Soc. Tasm. 133(1): 103-113. ISSN 0080-4703. School of Geography and Environmental Studies, University ofTasmania, GPO Box 252-78, Hobart, Tasmania, Australia 7001. The Huntingfield area has a varied vegetation, including substantial areas ofEucalyptus amygdalina heathy woodland, heath, buttongrass moorland and E. amygdalina shrubbyforest, with smaller areas ofwetland, grassland and E. ovata shrubbyforest. Six floristic communities are described for the area. Two hundred and one native vascular plant taxa, 26 moss species and ten liverworts are known from the area, which is particularly rich in orchids, two ofwhich are rare in Tasmania. Four other plant species are known to be rare and/or unreserved inTasmania. Sixty-four exotic plantspecies have been observed in the area, most ofwhich do not threaten the native biodiversity. However, a group offire-adapted shrubs are potentially serious invaders. Management problems in the area include the maintenance ofopen areas, weed invasion, pathogen invasion, introduced animals, fire, mechanised recreation, drainage from houses and roads, rubbish dumping and the gathering offirewood, sand and plants. Key Words: flora, forest, heath, Huntingfield, management, Tasmania, vegetation, wetland, woodland. INTRODUCTION species with the most cover in the shrub stratum (dominant species) was noted. If another species had more than half The Huntingfield Estate, approximately 400 ha of forest, the cover ofthe dominant one it was noted as a codominant. -
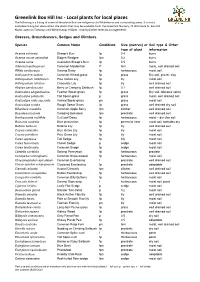
The Following Listing
Greenlink Box Hill Inc - Local plants for local places The following is a listing of some of the plants that are indigenous to Whitehorse and surrounding areas. It is not a complete listing but does reflect the plants that may be available from the Greenlink Nursery, 41 Wimmera St, Box Hill North, open on Tuesdays and Wednesdays 9:00am - midday (other times by arrangement). Grasses, Groundcovers, Sedges and Climbers Species Common Name Conditions Size (metres) or Soil type & Other type of plant information Acaena echinata Sheep’s Burr fp 0.4 burrs Acaena novae-zelandiae Bidgee Wedgee fpn 0.2 burrs Acaena ovina Australian Sheep’s Burr fp 0.5 burrs Adiantum aethiopicum Common Maidenhair P fern moist, well drained soil Allittia cardiocarpa Swamp Daisy fp herbaceous moist soil Anthosachne scabra Common Wheat-grass fp grass Dry soil, gravel, clay Arthropodium milleflorum Pale Vanilla Lily fp lily moist soil Arthropodium strictum Chocolate Lily fp lily well drained soil Atriplex semibaccata Berry or Creeping Saltbush fp 0.1 well drained soil Austrostipa elegantissima Feather Spear-grass fp grass Dry soil, tolerates saline Austrostipa pubinodis Tall Spear-grass p grass moist, well drained soil Austrostipa rudis ssp.rudis Veined Spear-grass pn grass moist soil Austrostipa scraba Rough Spear Grass fp grass well drained dry soil Billardiera mutabilis Common Apple Berry fpn climber well drained soil Bossiaea prostrata Creeping Bossiaea fpn prostrate well drained soil Brachyscome multifida Cut Leaf Daisy fp herbaceous moist – dry clay soil Brunonia -

Flora Surveys Introduction Survey Method Results
Hamish Saunders Memorial Island Survey Program 2009 45 Flora Surveys The most studied island is Sarah Results Island. This island has had several Introduction plans developed that have A total of 122 vascular flora included flora surveys but have species from 56 families were There have been few flora focused on the historical value of recorded across the islands surveys undertaken in the the island. The NVA holds some surveyed. The species are Macquarie Harbour area. Data on observations but the species list comprised of 50 higher plants the Natural Values Atlas (NVA) is not as comprehensive as that (7 monocots and 44 dicots) shows that observations for given in the plans. The Sarah and 13 lower plants. Of the this area are sourced from the Island Visitor Services Site Plan species recorded 14 are endemic Herbarium, projects undertaken (2006) cites a survey undertaken to Australia; 1 occurs only in by DPIPWE (or its predecessors) by Walsh (1992). The species Tasmania. Eighteen species are such as the Huon Pine Survey recorded for Sarah Island have considered to be primitive. There and the Millennium Seed Bank been added to some of the tables were 24 introduced species found Collection project. Other data in this report. with 9 of these being listed weeds. has been added to the NVA as One orchid species was found part of composite data sets such Survey Method that was not known to occur in as Tasforhab and wetforest data the south west of the state and the sources of which are not Botanical surveys were this discovery has considerably easily traceable. -

The Vegetation Communities Native Grassland
Edition 2 From Forest to Fjaeldmark The Vegetation Communities Native grassland Themeda australis Edition 2 From Forest to Fjaeldmark (revised - October 2017) 1 Native grassland Community (Code) Page Coastal grass and herbfield (GHC) 5 Highland Poa grassland (GPH) 7 Lowland grassland complex (GCL) 9 Lowland grassy sedgeland (GSL) 11 Lowland Poa labillardierei grassland (GPL) 12 Lowland Themeda triandra grassland (GTL) 14 Rockplate grassland (GRP) 16 General description align the key and the description of Lowland grassland complex ( ) with respect to the Native grasslands are defined as areas of native GCL required cover of native grass species. In 2017 vegetation dominated by native grasses with few or further minor revisions were made to improve no emergent woody species. Different types of information for general management issues, and to native grassland can be found in a variety of habitats, improve the description of Coastal grass and including coastal fore-dunes, dry slopes and valley herbfield (GHC) and its differentiation from Lowland bottoms, rock plates and subalpine flats. The lowland Poa labillardierei grassland (GPL). This is reflected in temperate grassland types have been recognised as the key to this Section. some of the most threatened vegetation communities in Australia. General management issues Some areas of native grassland are human-induced Most lowland native grassland in Tasmania has been and exist as a result of heavy burning, tree clearing cleared for agriculture since European settlement or dieback of the tree layer in grassy woodlands. (Barker 1999, Gilfedder 1990, Kirkpatrick et al. 1988, There are seven grassland communities recognised Kirkpatrick 1991, Williams et al. 2007). -
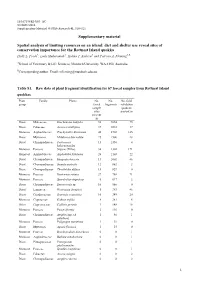
Supplementary Material Spatial Analysis of Limiting Resources on An
10.1071/WR14083_AC ©CSIRO 2014 Supplementary Material: Wildlife Research 41 , 510–521 Supplementary material Spatial analysis of limiting resources on an island: diet and shelter use reveal sites of conservation importance for the Rottnest Island quokka Holly L. Poole A, Laily Mukaromah A, Halina T. Kobryn A and Patricia A. Fleming A,B ASchool of Veterinary & Life Sciences, Murdoch University, WA 6150, Australia. BCorresponding author. Email: [email protected] Table S1. Raw data of plant fragment identification for 67 faecal samples from Rottnest Island quokkas Plant Family Plants No. No. No. field group faecal fragments validation sample quadrats sites present in present in Dicot Malvaceae Guichenotia ledifolia 52 9854 75 Dicot Fabaceae Acacia rostellifera 37 3018 37 Monocot Asphodelaceae Trachyandra divaricata 46 2702 145 Dicot Myrtaceae Melaleuca lanceolata 25 1506 28 Dicot Chenopodiaceae Tecticornia 13 1350 4 halocnemoides Monocot Poaceae Stipeae (Tribe) 34 1302 171 Monocot Asphodelaceae Asphodelus fistulosus 26 1103 22 Dicot Chenopodiaceae Rhagodia baccata 13 1002 46 Dicot Chenopodiaceae Suaeda australis 12 862 2 Dicot Chenopodiaceae Threlkeldia diffusa 15 829 0 Monocot Poaceae Rostraria cristata 27 788 71 Monocot Poaceae Sporobolus virginicus 5 617 2 Dicot Chenopodiaceae Sarcocornia sp . 10 560 0 Dicot Lamiaceae Westringia dampieri 5 383 46 Dicot Goodeniaceae Scaevola crassifolia 10 349 20 Monocot Cyperaceae Gahnia trifida 8 281 6 Other Cupressaceae Callitris preissii 3 148 18 Monocot Poaceae Poa poiformis 2 116 0 Dicot Chenopodiaceae Atriplex spp. (A. 1 40 1 paludosa ) Monocot Poaceae Polypogon maritimus 1 39 0 Dicot Myrtaceae Agonis flexuosa 1 15 0 Monocot Poaceae Brachypodium distachyon 0 0 1 Monocot Asphodelaceae Bulbine semibarbata 0 0 1 Dicot Pittosporaceae Pittosporum 0 0 1 phylliraeoides Monocot Poaceae Spinifex longifolius 0 0 1 Dicot Fabaceae Acacia saligna 0 0 2 Dicot Chenopodiaceae Atriplex cinerea 0 0 2 1 Dicot Asteraceae Centaurea sp . -

List of Plants
Indigenous Plant Nursery Plant Species List The following plant list contains some of the local native plants that may be available from the Edendale Indigenous Plant Nursery. Availability can vary so please contact the nursery for specific and seasonal availability of plants. Contact details: [email protected] Phone (03) 9433 3703 30 Gastons Road, Eltham VIC 3091 Open 7 days per week, 9.30am to 4.30pm Trees Species Common Name Size (height x width) Acacia dealbata Silver Wattle 6 – 30m x 5 – 10m Acacia implexa Lightwood 5 – 15m x 4 – 7m Acacia pycnantha Golden Wattle 3 – 10m x 2 – 5m Acacia mearnsii Black Wattle 8 – 25m x 6 – 10m Acacia melanoxylon Blackwood 5 – 30m x 4 – 15m Allocasuarina littoralis Black Sheoke 4 – 8m x 2 – 5m Allocasuarina verticillata Drooping Sheoke 4 – 11m x 3 – 6m Banksia marginata Silver Banksia 1 – 10m x 1 – 5m Callitris rhomboidea Oyster Bay Pine 9 – 15 m high Eucalyptus blakelyi Blakely’s Red Gum 15 – 24m x 10 – 15m Eucalyptus camaldulensis River Red Gum 15 – 50m x 15 – 35m Eucalyptus goniocalyx Long-leaved Box 8 – 20m x 6 – 15m Eucalyptus leucoxylon Yellow Gum 10 – 20m x 6 – 20m Eucalyptus macrorhyncha Red Stringybark 10 – 35m x 10 – 20m Eucalyptus melliodora Yellow Box 10 – 30m x 8 – 25m Eucalyptus ovata Swamp Gum 8 – 30m x 8 – 20m Eucalyptus pauciflora Snow Gum 8 – 12m x 6 – 10m Eucalyptus polyanthemos Red Box 7 – 25m x 5 – 15m Eucalyptus radiata Narrow-leaved Peppermint 10 – 30m x 6 – 20m Eucalyptus rubida Candlebark Gum 10 – 25m x 10 – 20m Eucalyptus tricarpa Red Ironbark 10 – 30m x -

Elwood Foreshore Biodiversity Constraints Assessment
Elwood Foreshore Biodiversity Constraints Assessment Port Phillip City Council © ECO LOGICAL AUSTRALIA PTY LTD 1 Elwood Foreshore Biodiversity Constraints Assessment | Port Phillip City Council DOCUMENT TRACKING Project Name Elwood Foreshore Biodiversity Constraints Assessment Project Number 15809 Project Manager Rani Sherriff Prepared by Rani Sherriff and Danielle Woodhams Reviewed by James Garden Approved by James Garden Status Draft Version Number V2 Last saved on 28 May 2020 This report should be cited as ‘Eco Logical Australia 2020. Elwood Foreshore Biodiversity Constraints Assessment. Prepared for Port Phillip City Council .’ ACKNOWLEDGEMENTS This document has been prepared by Eco Logical Australia Pty Ltd with support from Julian Hawkins. Disclaimer This document may only be used for the purpose for which it was commissioned and in accordance with the contract between Eco Logical Australia Pty Ltd and Port Phillip City Council. The scope of services was defined in consultation with Port Phillip City Council, by time and budgetary constraints imposed by the client, and the availability of reports and other data on the subject area. Changes to available information, legislation and schedules are made on an ongoing basis and readers should obtain up to date information. Eco Logical Australia Pty Ltd accepts no liability or responsibility whatsoever for or in respect of any use of or reliance upon this report and its supporting material by any third party. Information provided is not intended to be a substitute for site specific assessment or legal advice in relation to any matter. Unauthorised use of this report in any form is prohibited. Template 2.8.1 © ECO LOGICAL AUSTRALIA PTY LTD i Elwood Foreshore Biodiversity Constraints Assessment | Port Phillip City Council Contents 1. -

Gardens and Stewardship
GARDENS AND STEWARDSHIP Thaddeus Zagorski (Bachelor of Theology; Diploma of Education; Certificate 111 in Amenity Horticulture; Graduate Diploma in Environmental Studies with Honours) Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy October 2007 School of Geography and Environmental Studies University of Tasmania STATEMENT OF AUTHENTICITY This thesis contains no material which has been accepted for any other degree or graduate diploma by the University of Tasmania or in any other tertiary institution and, to the best of my knowledge and belief, this thesis contains no copy or paraphrase of material previously published or written by other persons, except where due acknowledgement is made in the text of the thesis or in footnotes. Thaddeus Zagorski University of Tasmania Date: This thesis may be made available for loan or limited copying in accordance with the Australian Copyright Act of 1968. Thaddeus Zagorski University of Tasmania Date: ACKNOWLEDGEMENTS This thesis is not merely the achievement of a personal goal, but a culmination of a journey that started many, many years ago. As culmination it is also an impetus to continue to that journey. In achieving this personal goal many people, supervisors, friends, family and University colleagues have been instrumental in contributing to the final product. The initial motivation and inspiration for me to start this study was given by Professor Jamie Kirkpatrick, Dr. Elaine Stratford, and my friend Alison Howman. For that challenge I thank you. I am deeply indebted to my three supervisors Professor Jamie Kirkpatrick, Dr. Elaine Stratford and Dr. Aidan Davison. Each in their individual, concerted and special way guided me to this omega point. -

Cunninghamia : a Journal of Plant Ecology for Eastern Australia
Cunninghamia 8(3): 2004 Heyligers & Adams, Flora and vegetation of Montagu Island 285 Flora and vegetation of Montagu Island – past and present Petrus C. Heyligers1 and Laurie G. Adams2 1 (CSIRO Sustainable Ecosystems, Queensland Biosciences Precinct, 306 Carmody Road, St. Lucia, Q 4067; 2Australian National Herbarium, CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601, AUSTRALIA. Corresponding author, email: [email protected] Abstract: Montagu Island (36°15’S; 150°14’E) is situated about 10 km east of Narooma on the New South Wales South Coast. The paper presents evidence about the changes in the terrestrial vegetation of the island since it was first seen by Europeans, provides a floristic inventory and gives a perspective on the effects of introduced species. Flinders (1814) mentions that the island ‘produced small trees.’ This is the only record of what grew on the island until in 1880 annotations on a map, made at the time of the construction of the lighthouse, mentioned the presence of scrub, trees and rank grass. This is confirmed by photographic evidence, but by 1932, when the botanist F. A. Rodway visited the island, the trees had disappeared. In 1973, during a land use survey of the South Coast, a team of CSIRO described the vegetation as a distinct series of dune communities belonging to the Lomandra longifolia – Pteridium esculentum – Phragmites australis complex. Vegetation mapping in the late 1980s confirmed the prevalence of these species, except that Pennisetum clandestinum then covered a large area along the west side of the island. Excluding taxa used for ornamental or culinary purposes, nearly 200 species of vascular plants have been recorded since 1932 of which about 140 were still present in the late 1990s.