Weathering Effects on the Structures of Mica-Type
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

NMAM 9000: Asbestos, Chrysotile By
ASBESTOS, CHRYSOTILE by XRD 9000 MW: ~283 CAS: 12001-29-5 RTECS: CI6478500 METHOD: 9000, Issue 3 EVALUATION: FULL Issue 1: 15 May 1989 Issue 3: 20 October 2015 EPA Standard (Bulk): 1% by weight PROPERTIES: Solid, fibrous mineral; conversion to forsterite at 580 °C; attacked by acids; loses water above 300 °C SYNONYMS: Chrysotile SAMPLING MEASUREMENT BULK TECHNIQUE: X-RAY POWDER DIFFRACTION SAMPLE: 1 g to 10 g ANALYTE: Chrysotile SHIPMENT: Seal securely to prevent escape of asbestos PREPARATION: Grind under liquid nitrogen; wet-sieve SAMPLE through 10 µm sieve STABILITY: Indefinitely DEPOSIT: 5 mg dust on 0.45 µm silver membrane BLANKS: None required filter ACCURACY XRD: Copper target X-ray tube; optimize for intensity; 1° slit; integrated intensity with RANGE STUDIED: 1% to 100% in talc [1] background subtraction BIAS: Negligible if standards and samples are CALIBRATION: Suspensions of asbestos in 2-propanol matched in particle size [1] RANGE: 1% to 100% asbestos OVERALL PRECISION ( ): Unknown; depends on matrix and ESTIMATED LOD: 0.2% asbestos in talc and calcite; 0.4% concentration asbestos in heavy X-ray absorbers such as ferric oxide ACCURACY: ±14% to ±25% PRECISION ( ): 0.07 (5% to 100% asbestos); 0.10 (@ 3% asbestos); 0.125 (@ 1% asbestos) APPLICABILITY: Analysis of percent chrysotile asbestos in bulk samples. INTERFERENCES: Antigorite (massive serpentine), chlorite, kaolinite, bementite, and brushite interfere. X-ray fluorescence and absorption is a problem with some elements; fluorescence can be circumvented with a diffracted beam monochromator, and absorption is corrected for in this method. OTHER METHODS: This is NIOSH method P&CAM 309 [2] applied to bulk samples only, since the sensitivity is not adequate for personal air samples. -

A108-316 (10/10/16)
American Industrial Hygiene Association Bulk Asbestos Proficiency Analytical Testing Program Results of Round A108-316 10/10/2016 John Herrock Laboratory ID Number Total Penalty Points 0 University of Louisiana, Monroe - Dept of 213022 Round Status P Toxicology Program Status P 700 University Ave. Monroe, LA 71209 UNITED STATES Lot Designation\Sample ID Numbers A) 1761 B) 2702 C) 1897 D) 4134 Analysis Results from Laboratory Number 213022 Asbestos (%) CHRY (3) ANTH(22) NONE CHRY (1) Other Fibrous Materials (%) FBGL (1) Nonfibrous Material (%) ACID (52) OTHR (55) ACID (60) OTHR (60) MICA (33) MICA (11) OTHR (38) ACID (29) Penalty Points Assessed 0 0 0 0 Analysis Results from Reference Laboratory One Asbestos (%) CHRY(5.8) ANTH (12) NONE CHRY (3.8) ACTN (0.1) Other Fibrous Materials (%) CELL (0.1) OTHR *1 (0.1) CELL (1) Nonfibrous Material (%) MICA (45) OTHR *2(87.9) OTHR *3 (35) PERL (20) CASO (49) OTHR *4 (65) OTHR *5 (20) OTHR *6 (55.2) Analysis Results from Reference Laboratory Two Asbestos (%) CHRY (2.5) ANTH (28) (0) CHRY(3.5%) TREM(trace) Other Fibrous Materials (%) FBGL (trace) Nonfibrous Material (%) OTHR *7 (60) OTHR *9 (24) OTHR *11 (80) OTHR *14 (20) OTHR *8(37.5) OTHR *10 (48) OTHR *12 (18) OTHR *15(76.5) OTHR *13 (2) Analysis Results from RTI International Asbestos (%) CHRY (4) ANTH (28) NONE CHRY (3) ACTN (Tra) Other Fibrous Materials (%) OTHR *16(Tra) POLY (Tra) CELL (1) OTHR *17(Tra) Nonfibrous Material (%) MICA (29) OTHR *18 (53) CACO (89) OTHR *22 (28) CASO (67) OTHR *19 (19) OTHR *20 (9) PERL (45) OTHR *21 (2) OTHR *23 -

40 Common Minerals and Their Uses
40 Common Minerals and Their Uses Aluminum Beryllium The most abundant metal element in Earth’s Used in the nuclear industry and to crust. Aluminum originates as an oxide called make light, very strong alloys used in the alumina. Bauxite ore is the main source aircraft industry. Beryllium salts are used of aluminum and must be imported from in fluorescent lamps, in X-ray tubes and as Jamaica, Guinea, Brazil, Guyana, etc. Used a deoxidizer in bronze metallurgy. Beryl is in transportation (automobiles), packaging, the gem stones emerald and aquamarine. It building/construction, electrical, machinery is used in computers, telecommunication and other uses. The U.S. was 100 percent products, aerospace and defense import reliant for its aluminum in 2012. applications, appliances and automotive and consumer electronics. Also used in medical Antimony equipment. The U.S. was 10 percent import A native element; antimony metal is reliant in 2012. extracted from stibnite ore and other minerals. Used as a hardening alloy for Chromite lead, especially storage batteries and cable The U.S. consumes about 6 percent of world sheaths; also used in bearing metal, type chromite ore production in various forms metal, solder, collapsible tubes and foil, sheet of imported materials, such as chromite ore, and pipes and semiconductor technology. chromite chemicals, chromium ferroalloys, Antimony is used as a flame retardant, in chromium metal and stainless steel. Used fireworks, and in antimony salts are used in as an alloy and in stainless and heat resisting the rubber, chemical and textile industries, steel products. Used in chemical and as well as medicine and glassmaking. -

Clay Minerals Soils to Engineering Technology to Cat Litter
Clay Minerals Soils to Engineering Technology to Cat Litter USC Mineralogy Geol 215a (Anderson) Clay Minerals Clay minerals likely are the most utilized minerals … not just as the soils that grow plants for foods and garment, but a great range of applications, including oil absorbants, iron casting, animal feeds, pottery, china, pharmaceuticals, drilling fluids, waste water treatment, food preparation, paint, and … yes, cat litter! Bentonite workings, WY Clay Minerals There are three main groups of clay minerals: Kaolinite - also includes dickite and nacrite; formed by the decomposition of orthoclase feldspar (e.g. in granite); kaolin is the principal constituent in china clay. Illite - also includes glauconite (a green clay sand) and are the commonest clay minerals; formed by the decomposition of some micas and feldspars; predominant in marine clays and shales. Smectites or montmorillonites - also includes bentonite and vermiculite; formed by the alteration of mafic igneous rocks rich in Ca and Mg; weak linkage by cations (e.g. Na+, Ca++) results in high swelling/shrinking potential Clay Minerals are Phyllosilicates All have layers of Si tetrahedra SEM view of clay and layers of Al, Fe, Mg octahedra, similar to gibbsite or brucite Clay Minerals The kaolinite clays are 1:1 phyllosilicates The montmorillonite and illite clays are 2:1 phyllosilicates 1:1 and 2:1 Clay Minerals Marine Clays Clays mostly form on land but are often transported to the oceans, covering vast regions. Kaolinite Al2Si2O5(OH)2 Kaolinite clays have long been used in the ceramic industry, especially in fine porcelains, because they can be easily molded, have a fine texture, and are white when fired. -

A RARE-ALKALI BIOTITE from KINGS MOUNTAIN, NORTH CAROLINA1 Fnanr L
A RARE-ALKALI BIOTITE FROM KINGS MOUNTAIN, NORTH CAROLINA1 FnaNr L. Hnss2 arqn Ror-r.rx E. SrrvrNs3 Severalyears ago, after Judge Harry E. Way of Custer, South Dakota, had spectroscopically detected the rare-alkali metals in a deep-brown mica from a pegmatite containing pollucite and lithium minerals, in Tin Mountain, 7 miles west of Custer, another brown mica was collected, which had developed notably in mica schist at its contact with a similar mass of pegmatite about one half mile east of Tin Mountai". J. J. Fahey of the United States GeolgoicalSurvey analyzed the mica, and it proved to contain the rare-alkali metalsaand to be considerably difierent from any mica theretofore described. Although the cesium-bearing minerals before known (pollucite, lepidolite, and beryl) had come from the zone of highest temperature in the pegmatite, the brown mica was from the zone of lowest temperature. The occurrence naturally suggestedthat where dark mica was found developed at the border of a pegmatite, especially one carrying lithium minerals, it should be examined for the rare-alkali metals. As had been found by Judge Way, spectroscopictests on the biotite from Tin Moun- tain gave strong lithium and rubidium lines, and faint cesium lines. Lithium lines were shown in a biotite from the border of the Morefield pegmatite, a mile south of Winterham, Virginia, but rubidium and cesium w'erenot detected. $imilarly placed dark micas from Newry and Hodgeon HiII, near Buckfield, Maine, gave negative results. They should be retested. Tests by Dr. Charles E. White on a shiny dark mica from the Chestnut FIat pegmatite near Spruce Pine, North Carolina, gave strong lithium and weaker cesium lines. -
Mica Data Sheet
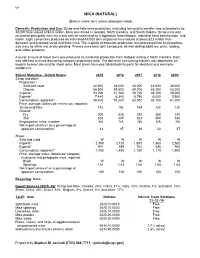
108 MICA (NATURAL) (Data in metric tons unless otherwise noted) Domestic Production and Use: Scrap and flake mica production, excluding low-quality sericite, was estimated to be 38,000 tons valued at $4.6 million. Mica was mined in Georgia, North Carolina, and South Dakota. Scrap mica was recovered principally from mica and sericite schist and as a byproduct from feldspar, industrial sand beneficiation, and kaolin. Eight companies produced an estimated 63,000 tons of ground mica valued at about $22 million from domestic and imported scrap and flake mica. The majority of domestic production was processed into small-particle- size mica by either wet or dry grinding. Primary uses were joint compound, oil-well-drilling additives, paint, roofing, and rubber products. A minor amount of sheet mica was produced as incidental production from feldspar mining in North Carolina. Data was withheld to avoid disclosing company proprietary data. The domestic consuming industry was dependent on imports to meet demand for sheet mica. Most sheet mica was fabricated into parts for electrical and electronic equipment. Salient Statistics—United States: 2015 2016 2017 2018 2019e Scrap and flake: Production:1 Sold and used 32,600 28,000 40,000 44,000 38,000 Ground 65,800 59,500 69,700 65,300 63,000 Imports2 33,200 31,500 29,700 28,100 29,000 Exports3 7,440 6,340 6,790 6,000 5,900 Consumption, apparent4 58,400 53,200 62,900 66,100 61,000 Price, average, dollars per metric ton, reported: Scrap and flake 142 152 165 122 120 Ground: Dry 305 320 292 308 310 Wet 423 435 424 454 480 Employment, mine, number NA NA NA NA NA Net import reliance5 as a percentage of apparent consumption 44 47 36 33 37 Sheet: Sold and used W W W W W Imports6 2,390 2,120 1,850 1,860 2,500 Exports7 911 689 704 686 950 Consumption, apparent5 1,480 1,430 1,150 1,170 1,600 Price, average value, dollars per kilogram, muscovite and phlogopite mica, reported: Block W W W W W Splittings 1.61 1.61 1.66 1.65 1.65 Net import reliance5 as a percentage of apparent consumption 100 100 100 100 100 Recycling: None. -

Rock and Mineral Identification for Engineers
Rock and Mineral Identification for Engineers November 1991 r~ u.s. Department of Transportation Federal Highway Administration acid bottle 8 granite ~~_k_nife _) v / muscovite 8 magnify~in_g . lens~ 0 09<2) Some common rocks, minerals, and identification aids (see text). Rock And Mineral Identification for Engineers TABLE OF CONTENTS Introduction ................................................................................ 1 Minerals ...................................................................................... 2 Rocks ........................................................................................... 6 Mineral Identification Procedure ............................................ 8 Rock Identification Procedure ............................................... 22 Engineering Properties of Rock Types ................................. 42 Summary ................................................................................... 49 Appendix: References ............................................................. 50 FIGURES 1. Moh's Hardness Scale ......................................................... 10 2. The Mineral Chert ............................................................... 16 3. The Mineral Quartz ............................................................. 16 4. The Mineral Plagioclase ...................................................... 17 5. The Minerals Orthoclase ..................................................... 17 6. The Mineral Hornblende ................................................... -

Did Biology Emerge from Biotite in Micaceous Clay? H
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 17 September 2020 doi:10.20944/preprints202009.0409.v1 Article Did Biology Emerge from Biotite in Micaceous Clay? H. Greenwood Hansma1* Physics Department, University of California, Santa Barbara, CA; [email protected] 1 Physics Department, University of California, Santa Barbara, CA; [email protected] * Correspondence: [email protected] Received: date; Accepted: date; Published: date Abstract: An origin of life between the sheets of micaceous clay is proposed to involve the following steps: 1) evolution of metabolic cycles and nucleic acid replication, in separate niches in biotite mica; 2) evolution of protein synthesis on ribosomes formed by liquid-in-liquid phase separation; 3) repeated encapsulation by membranes of molecules required for the metabolic cycles, replication, and protein synthesis; 4) interactions and fusion of the these membranes containing enclosed molecules; resulting eventually in 5) an occasional living cell, containing everything necessary for life. The spaces between mica sheets have many strengths as a site for life’s origins: mechanochemistry and wet-dry cycles as energy sources, an 0.5-nm anionic crystal lattice with potassium counterions (K+), hydrogen-bonding, enclosure, and more. Mica pieces in micaceous clay are large enough to support mechanochemistry from moving mica sheets. Biotite mica is an iron- rich mica capable of redox reactions, where the stages of life’s origins could have occurred, in micaceous clay. Keywords: clay; mica; biotite; muscovite; origin of life; origins of life; mechanical energy; work; wet- dry cycles 1. Introduction Somewhere there was a habitat, hospitable for everything needed for the origins of life. -

What We Know About Subduction Zones from the Metamorphic Rock Record
What we know about subduction zones from the metamorphic rock record Sarah Penniston-Dorland University of Maryland Subduction zones are complex We can learn a lot about processes occurring within active subduction zones by analysis of metamorphic rocks exhumed from ancient subduction zones Accreonary prism • Rocks are exhumed from a wide range of different parts of subduction zones. • Exhumed rocks from fossil subduction zones tell us about materials, conditions and processes within subduction zones • They provide complementary information to observations from active subduction systems Tatsumi, 2005 The subduction interface is more complex than we usually draw Mélange (Bebout, and Penniston-Dorland, 2015) Information from exhumed metamorphic rocks 1. Thermal structure The minerals in exhumed rocks of the subducted slab provide information about the thermal structure of subduction zones. 2. Fluids Metamorphism generates fluids. Fossil subduction zones preserve records of fluid-related processes. 3. Rheology and deformation Rocks from fossil subduction zones record deformation histories and provide information about the nature of the interface and the physical properties of rocks at the interface. 4. Geochemical cycling Metamorphism of the subducting slab plays a key role in the cycling of various elements through subduction zones. Thermal structure Equilibrium Thermodynamics provides the basis for estimating P-T conditions using mineral assemblages and compositions Systems act to minimize Gibbs Free Energy (chemical potential energy) Metamorphic facies and tectonic environment SubduconSubducon zone metamorphism zone metamorphism Regional metamorphism during collision Mid-ocean ridge metamorphism Contact metamorphism around plutons Determining P-T conditions from metamorphic rocks Assumption of chemical equilibrium Classic thermobarometry Based on equilibrium reactions for minerals in rocks, uses the compositions of those minerals and their thermodynamic properties e.g. -

Geology Tour Glossary
GEOLOGY TOUR GLOSSARY ABRASION - a form of mechanical weathering involving the scraping of a rock surface by friction between rocks and moving particles during their transport by wind, glaciers, waves, gravity, running water, or erosion BIOLOGICAL WEATHERING – a type of chemical weathering in which biologically produced chemicals breakdown rocks, soils and minerals BIOTITE - a common dark-brown, dark-green, or black mineral of the mica group CHEMICAL WEATHERING - the direct effect of atmospheric and/or biological chemicals on the breakdown of rocks, soils and minerals COUNTRY ROCK - rock that is native to an area EXFOLIATION - the process in which rocks weather by peeling off in sheets rather that eroding grain by grain FALL ZONE - the geomorphologic break between an upland region of relatively hard crystalline basement rock and a coastal plain of softer sedimentary rock; distinguished by a drop in elevation and waterfalls in rivers FAULT - a planar fracture or discontinuity in a volume of rock, across which there has been significant displacement along the fractures as a result of earth movement FELDSPAR - an abundant, rock-forming mineral that varies in color from pink, yellow-orange, tan-white. Large bits often have squared edges. About 60 percent of the Earth's outer crust is composed of feldspar GEOLOGY - the study of the history and structure of the Earth, the rocks that the Earth is made of, and the processes that form and change the rocks GNEISSIC BANDING - a type of foliation in metamorphic rock consisting of roughly parallel dark and light bands of rock GRANITE - a hard, granular, igneous rock, formed as magma solidifies far below the earth’s surface. -

AN INTERSTRATIFIED MIXTURE of MICA CLAY MINERALS Susuuu Snrnooeanp Tosnro Suoo,Tokyo Uniaersityof Etlucation,T Okyo,J Apan
1960 THE AMERICAN MINERALOGIST, VOL 45, SEPTE\'IBER-OCTOBER' AN INTERSTRATIFIED MIXTURE OF MICA CLAY MINERALS Susuuu SnrnooeaNp Tosnro Suoo,Tokyo Uniaersityof Etlucation,T okYo,J aPan. Assrn.{ct INrtouucuoN found almost free from impurities. Thus such mineralsare not so Iare as ap- we have hitherto anticipated, and the present mineral is considered propriate for the description of its mineralogical and crystallographic properties. MopB ol OccuRRENCE 1O7O S. SHIMODA AND 7.. SUDO complexmineral yonago associations.The mine now being consideredis one of the important pyrophyllite-diaspore depositsin Japan. The area near the ore deposit consists of fine grained sandstoneand shaleintruded by porphyrite and andesitedykes. A[ of theserocks are covered by a lava flow. The ore deposit occursalong the boundary zone between the porphyrite and andesitedykes repracingboth of theserocks. The ore bodies consist of diaspore,cray minerals, quartz and pyrite. Diaspore occurs as compact or powdery massesmostly cemented by pyrophyllite. About eighty specimenswere coilectedin the area inciuding the ore deposit, and the amounts of the minerals in each specimenwere esti- mated by r-ray analysesusing an internal standard of fluorite. standard curves were made using pure clay mineralsfrom the other locaiities.The confirmed mineralsare diaspore,pyrophyllite, quartz,sericite, kaoiinite, halloysite, montmorillonite, and pyrite. By plotting the result of the qualitative estimationsof these minerals except pyrite, zonar distribu- tions were confirmed particularly with regard to ttre distributions of diaspore,pyrophyllite, and kaolin minerals.The center of the zonal dis- tribution was confirmed in two difierent places; in both of which the zonal distributions were found to be (diaspore and pyrophyllite)_ (kaolin minerals)-( quaftz), going progressively u*uy fro- ih" ."rrr".