Pharma/Biotech M&A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Scientific Organizing Committee Gratefully Acknowledges the Pharmaceutical and Biotechnology Industry for Their Generous Support of WCBP 2019
The Scientific Organizing Committee gratefully acknowledges the pharmaceutical and biotechnology industry for their generous support of WCBP 2019: Strategic Diamond Program Partners F. Hoffmann-La Roche Ltd. Genentech, a Member of the Roche Group Strategic Platinum Program Partners Amgen Inc. Biogen MedImmune, A member of the AstraZeneca Group Strategic Gold Program Partners Eli Lilly and Company Merck & Co., Inc. Novo Nordisk A/S Pfizer, Inc. Strategic Silver Program Partner Sanofi 1 Gold Program Partner Bill and Melinda Gates Foundation Silver Program Partners BioMarin Pharmaceutical Inc. Bristol-Myers Squibb Company GlaxoSmithKline Jazz Pharmaceuticals Bronze Program Partner Seattle Genetics, Inc. Friend of CASSS Janssen R&D, LLC 2 The Scientific Organizing Committee gratefully acknowledges the Program Partners and Exhibitors for their generous support of WCBP 2019: Diamond Program Partners Agilent Technologies Catalent Pharma Solutions Eurofins Pharma Discovery Services ProteinSimple, a Bio-Techne brand Thermo Fisher Scientific Waters Corporation Platinum Program Partners BioAnalytix SCIEX Bronze Program Partner Bruker Corporation 3 The Scientific Organizing Committee gratefully acknowledges the Exhibitors for their generous support of WCBP 2019: Exhibitor Partners 908 Devices Inc. NanoImaging Services Agilent Technologies New England Biolabs Associates of Cape Cod, Inc. Postnova Analytics Inc. BioAnalytix Inc. Pressure BioSciences Inc. Bruker Corporation Protein Metrics, Inc. Catalent Pharma Solutions ProteinSimple, a Bio-Techne brand Charles River Laboratories ProZyme, A part of Agilent Covance, Inc. RedShift BioAnalytics, Inc. Cygnus Technologies Rockland Immunochemicals, Inc. Envigo SCIEX Eurofins BioPharma Product Testing SGS Life Science Services Eurofins Pharma Discovery Services Shimadzu Scientific Instruments, Inc. FortéBio - Biologics by Molecular Devices Thermo Fisher Scientific GE Healthcare Life Sciences U.S. -

Fibrolase: Trials and Tribulations
Toxins 2010, 2, 793-808; doi:10.3390/toxins2040793 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Review Fibrolase: Trials and Tribulations Francis S. Markland 1,2,* and Steve Swenson 1,2 1 Department of Biochemistry and Molecular Biology, Cancer Research Laboratory, Keck School of Medicine, University of Southern California, 1303 N. Mission Rd., Los Angeles, CA 90033, USA 2 USC/Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-(323) 224-7981; Fax: +1-(323) 224-7679. Received: 11 March 2010; in revised form: 31 March 2010 / Accepted: 19 April 2010 / Published: 20 April 2010 Abstract: Fibrolase is the fibrinolytic enzyme isolated from Agkistrodon contortrix contortrix (southern copperhead snake) venom. The enzyme was purified by a three-step HPLC procedure and was shown to be homogeneous by standard criteria including reverse phase HPLC, molecular sieve chromatography and SDS-PAGE. The purified enzyme is a zinc metalloproteinase containing one mole of zinc. It is composed of 203 amino acids with a blocked amino-terminus due to cyclization of the terminal Gln residue. Fibrolase shares a significant degree of homology with enzymes of the reprolysin sub-family of metalloproteinases including an active site homology of close to 100%; it is rapidly inhibited by chelating agents such as EDTA, and by alpha2-macroglobulin (). The enzyme is a direct-acting thrombolytic agent and does not rely on plasminogen for clot dissolution. Fibrolase rapidly cleaves the A()-chain of fibrinogen and the B()-chain at a slower rate; it has no activity on the -chain. -

Guidelines on CVD During Pregnancy (TF17) - Task Force Members and Additional Contributors
Guidelines on CVD during Pregnancy (TF17) - Task Force Members and Additional Contributors Expert Relationship Type of relationship with industry Financial declaration with Industry Blomstrom-Lundqvist Yes A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. Carina - Medtronic : Pacemaker B - Payment to your Institution: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Atricure : AF ablation Borghi Claudio Yes A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Menarini International : ACE-inhibitors - Boheringer Ingelheim : Antihypertensive drugs - Recordati International : Antihypertensive drugs - Novartis : Antihypertensive, Heart Failure D - Research funding (departmental or institutional). - Barilla Food Company : Lactotripeptides Cifkova Renata Yes A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Boehringer-Ingelheim : cardiovascular - Daiichi Sankyo : cardiovascular - MSD-SP, Boehringer Ingelheim, Bayer : cardiovascular B - Payment to your Institution: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Daiichi Sankyo : cardiovascular - Novartis : cardiovascular D - Research funding (departmental or institutional). - Krka Czech Republic : cardiovascular - Servier Czech Republic : cardiovascular Ferreira J Rafael No -

Coordination Des Syndicats CGT
Coordination des syndicats CGT STRATEGIE DE LA DIRECTION DU GROUPE SANOFI CONSEQUENCES INDUSTRIELLES ET SOCIALES Document d’août 2014 1. Situation économique – Coût du capital p2 2. Evolution des effectifs – Bilan des restructurations majeures p3 3. Stratégie Sanofi 2009-2015 : Désengagement scientifique et industriel en Europe et plus particulièrement en France p4 4. Stratégie de structuration du groupe en entités qui peuvent être cédées, vendues, fermées, échangées. p6 5. Crédit d’impôt – Des aides publiques pour quel usage ? p7 6. Industrie pharmaceutique : des besoins fondamentaux p7 7. Interpellation des élus et du gouvernement p8 1. Situation économique – Coût du capital Première entreprise pharmaceutique française et européenne. Sanofi est issu de la fusion de nombreux laboratoires pharmaceutiques français dont les principaux étaient Roussel Uclaf, Rhône Poulenc, Synthelabo, Sanofi et de l’allemand Hoechst. Sanofi représente 30 à 40% du potentiel national (effectifs, sites, R&D,…) de l’industrie pharmaceutique française dans notre pays. L’avenir du groupe et de ses activités en France conditionne l’avenir de l’industrie pharmaceutique française et constitue un élément incontournable de l’indépendance thérapeutique du pays. Le C.A. de sanofi dans le monde sur 2013 a atteint 33 milliards € et devrait se situer à un niveau légèrement supérieur en 2014. Plusieurs médicaments de référence étant aujourd’hui tombés dans le domaine public, le chiffre d’affaires repart à la hausse. Le résultat net des activités a été de 6,8 milliards € en 2013 et les projections sur 2014 laissent envisager une progression de 5% de celui-ci. La rentabilité est estimée par les économistes parmi les meilleures de l’industrie pharmaceutique dans le monde. -

The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry
The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry By: Maitri Punjabi Honors Thesis Economics Department The University of North Carolina at Chapel Hill March 2016 Approved: ______________________________ Dr. Jonathan Williams Punjabi 2 Abstract This paper analyzes the effect of the changing nature of innovation on pharmaceutical firm market value from the years 1987 to 2010 by using U.S. patent and claim data. Over the years, firms have started shifting focus from primary innovation to secondary innovation as new ideas and new compounds become more difficult to generate. In this study, we analyze the impact of this patent portfolio shift on the market capitalization of pharmaceutical firms. After using firm fixed effects and the instrumental variable approach, we find that there exists a strong positive relationship between secondary innovations and the market value of the firm– in fact, we find a stronger relationship than is observed between primary innovation and market value. When focusing on the different levels of innovation within the industry, we find that this relationship is stronger for less-innovative firms (those that have produced fewer patents) than it is for highly- innovative firms. We also find that this relationship is stronger for firms that spend less on research and development, complementing earlier findings that research productivity is declining over time. Punjabi 3 Acknowledgements I would primarily like to thank my adviser, Dr. Jonathan Williams, for his patience and constant support. Without his kind and helpful attitude, this project would have been a much more frustrating process. Through his knowledge of the industry, I have gained valuable insight and have learned a great deal about a unique and growing field. -
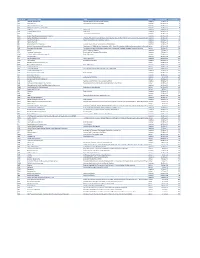
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

United States Securities and Exchange Commission Form 10-K Shire Pharmaceuticals Group
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ፤ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 អ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 0-29630 SHIRE PHARMACEUTICALS GROUP PLC (Exact name of registrant as specified in its charter) England and Wales (State or other jurisdiction (I.R.S. Employer of incorporation or organization) Identification No.) N.A. East Anton, Andover, Hampshire SP10 5RG England (Address of principal executive offices) (Zip Code) 44 1264 333455 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of exchange on which registered American Depository Shares, each representing Nasdaq National Market 3 Ordinary Shares, 5 pence nominal value per share Securities registered pursuant to Section 12(g) of the Act: None (Title of class) Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ፤ No អ Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference to Part III of this Form 10-K or any amendment to this Form 10-K. -

Ce Que Sanofi Dit De La Politique Industrielle Française
Ce que Sanofi dit de la politique industrielle française mediapart.fr/journal/economie/030221/ce-que-sanofi-dit-de-la-politique-industrielle-francaise Martine Orange, Mediapart, 3 février 2021 Les salariés de Sanofi ont beau essayer de chercher des explications, ils ne comprennent pas. Ou plutôt ils ne comprennent que trop bien la conduite du groupe pharmaceutique. Après le revers de sa stratégie dans l’élaboration d’un vaccin contre le Covid-19, repoussé désormais au mieux à la fin de l’année, tout aurait dû pousser la direction de Sanofi à s’interroger sur la pertinence de ses choix, sur la place laissée à la recherche jugée comme essentielle. Mais rien ne s’est passé. Le 28 janvier, la direction de Sanofi Recherche et Développement en France a confirmé à l’occasion d’un comité social d’entreprise (CSE) la suppression de 364 emplois en France, une mesure qui vise particulièrement l’unité de Strasbourg appelée à être transférée en région parisienne. Ce plan s’inscrit dans un programme plus large annoncé en juillet 2020. Le groupe entend supprimer 1 700 emplois en Europe dont un millier en France sur trois ans. « Mais ce n’est qu’une partie du projet Pluton, prévient Jean-Louis Perrin, délégué CGT à Montpellier. Sanofi est en train de se désindustrialiser. Toute la pharmacie de synthèse est appelée à disparaître dans le groupe. Les sites de Sisteron, Elbeuf, Vertolaye, Brindisi (Italie), Francfort (Allemagne), Haverhill (Royaume-Uni), Újpest (Hongrie) sont destinés à sortir du groupe. Au total, cela représente 3 500 emplois. » Centre de distribution de Sanofi à Val-de-Reuil. -

Fusiones 20De 20Labo
"Cuando los grandes se hacen gigantes" Fusiones de Laboratorios 1 INDICE PRÓLOGO……………………………………………………………………………...…….. 3 INTRODUCCIÓN: FUSIONES Y ADQUISICIONES…………………………………..….. 4 INVESTIGACIÓN Y DESARROLLO………………………………………………………. 5 LA INDUSTRIA FARMACEUTICA………………………………………………………. 16 FUSIÓN SANOFI – AVENTIS…………………………………………………………...… 20 FUSIÓN BAYER – SCHERING……………………………………………………………. 26 FUSIÓN PFIZER – WYETH……………………………………………………………….. 30 FUSIÓN MERCK & CO. – SCHERING PLOUGH…………………………………..……. 31 FUSIÓN ROCHE – GENENTECH………………………………………………………… 34 MERCADOS REGIONALES………………………………………………………….…… 40 LA INDUSTRIA FARMACÉUTICA EN LA ARGENTINA……………………...………. 42 CONCLUSIÓN……………………………………………………………………………… 49 2 PRÓLOGO Es un verdadero privilegio que hayan pensado en mí para prologar este interesante trabajo relacionado con la formación profesional de estos inquietos alumnos de la Carrera de Agentes de Propaganda Médica. Grato además, pues recrea mi participación activa en el mundo de la Industria Farmacéutica, en calidad de Asesor de la Fuerza de Ventas e Investigador Principal durante varias décadas, período que fue enriquecedor para mí y sumó un importante valor agregado a mi bagaje médico y personal. Entiendo que lo sucedido en los avances científicos y tecnológicos durante los últimos cuarenta años, ha constituido un quiebre en la Historia de la Medicina y, por lo tanto, de la Humanidad. Es por ello que veo con beneplácito que la formación de estos entusiastas jóvenes va de la mano con los cambios de planes y esquemas de estudios de los futuros médicos. -

The Life of the Abortion Pill in the United States
The Life of the Abortion Pill in the United States The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation The Life of the Abortion Pill in the United States (2000 Third Year Paper) Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:8852153 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA 80 The Life of the Abortion Pill in the United States Julie A. Hogan Eleven years after mifepristone1, the drug that chemically induces abortion and hence coined the abortion pill, was approved for use in France, American women still do not have access to the drug, although women in at least ten other nations do.2 In 1988, Americans thought the Abortion Pill [was] on the Hori- zon.3 In 1993, almost five years later, American women still did not have access to the drug, although many women's hopes were raised by newspaper headlines claiming that the Door May Be Open for [the] Abortion Pill to Be Sold in [the] U.S.4 and newspaper accounts predicting that mifepristone would be available in the United States in 1996.5 In 1996, the headlines reported that the Approval of [the] Abortion Pill by the FDA [was] Likely Soon.6 Yet, mifepristone was still not available in 1999, and newspaper headlines were less optimistic about pre- 1Mifepristone is the generic name for RU-486, the designation given the drug by its French maker, Roussel-Uclaf. -

Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500
Dyax Corp. 300 Technology Square Cambridge, MA 02139 617 225-2500 www.dyax.com Other Offices Dyax SA, Liege, Belgium ADVANCING NOVEL THERAPEUTIC PRODUCTS Dyax Corp. Annual Report 2003 Corporate Information Dyax Achievements 2003 Dyax Goals 2004 Directors Executive Officers and Stock Listing Henry E. Blair Key Employees Common stock has been traded on the Nasdaq Stock Market Advances in Clinical Development Clinical Development Chairman, President and Henry E. Blair* under the symbol DYAX since our initial public offering in Milestones Chief Executive Officer, Chairman, President and August 14, 2000. DX-88/Hereditary Angioedema Dyax Corp. Chief Executive Officer DX-88/Hereditary Constantine E. Stephen S. Galliker, CPA* The following table gives the quarterly high and low sales G Completed 9-patient Phase II trial, met primary endpoints Angioedema Anagnostopoulos, Ph.D. EVP Finance and prices of our common stock for the last three years. G Genzyme Corporation joined Dyax as joint venture partner Managing General Partner, Administration and G Complete Phase II 2001 2002 2003 G Gateway Associates, LP Chief Financial Officer Completed 3 of 4 dose cohorts in 48-patient Phase II EDEMA1 trial EDEMA1 study High Low High Low High Low G Susan B. Bayh, J.D. Lynn G. Baird, Ph.D.* Initiated Phase II EDEMA2 trial G Periodically observe James W. Fordyce SVP Development First Quarter $20.94 $6.56 $11.38 $3.10 $2.25 $1.52 G Orphan Drug designation granted in U.S. and Europe effects of repeat dosing in Phase II EDEMA2 Managing Partner, Robert C. Ladner, Ph.D. Second Quarter $19.99 $6.81 $ 4.68 $3.20 $4.90 $1.67 Fordyce & Gabrielson, LLC SVP and Chief DX-88/On-Pump Open Heart Surgery (CABG) study Third Quarter $21.24 $6.05 $ 4.20 $1.65 $7.50 $2.58 Mary Ann Gray, Ph.D. -

In Re Nuvelo, Inc. Securities Litigation 07-CV-04056-Declaration of Mark
BERGER & MONTAGUE, P.C. Sherrie R. Savett Carole A. Broderick Phyllis M. Parker 1622 Locust Street Philadelphia, PA 19103 Tel: (215) 875-3000 Fax: (215) 875-4604 Email: [email protected] cbroderick@bm ,net [email protected] IZARD NOBEL LLP ROBBINS GELLER RUDMAN Jeffrey S. Nobel & DOWD LLP Mark P. Kindall, Bar No 138703 Darren J. Robbins Nancy A. Kulesa Dennis J. Herman 29 South Main Street Eli R. Greenstein Suite 215 S. Ashar Ahmed West Hartford, Ct 06107 Post-Montgomery Center Tel: (860) 493-6292 One Montgomery Street, Suite 1800 Fax: (860) 493-6290 San Francisco, CA 94104 Email: [email protected] Tel: (415) 288-4545 [email protected] Fax: (415) 288-4534 [email protected] Email: [email protected] dennisb@rgrdlaw,corn [email protected] aahmed@rgrdlaw com Co-Lead Counsel for Plaintiffs Liaison Counsel UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN FRANCISCO DIVISION In re NUVELO, INC. SECURITIES Master File No 07-CV-04056-VRW LITIGATION CLASS ACTION DECLARATION OF MARK P. KINDALL IN SUPPORT OF PLAINTIFFS' MOTION FOR CLASS CERTIFICATION DATE: March .3, 2011 I TIME: 10:00 a.rn. I COURTROOM: 6 DECLARATION OF MARK P KINDALL IN SUPPORT OF PLArNTIFFS' MOTION FOR CLASS CERTIFICATION - 07-CV-04056-VRW DECLARATION OF MARK P. KINDALL IN SUPPORT OF PLAINTIFFS' MOTION FOR CLASS CERTIFICATION 2 3 I, Mark P. Kindall, hereby declare as follows: 4 1. I am a partner at the law firm of Izard Nobel LLP, which was appointed Co-Lead 5 Counsel for Plaintiffs in this litigation on September 19, 2007. I have personal knowledge of the 6 facts set forth herein.