Thesis Gap Analysis of India's Western
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shankar Ias Academy Test 18 - Geography - Full Test - Answer Key
SHANKAR IAS ACADEMY TEST 18 - GEOGRAPHY - FULL TEST - ANSWER KEY 1. Ans (a) Explanation: Soil found in Tropical deciduous forest rich in nutrients. 2. Ans (b) Explanation: Sea breeze is caused due to the heating of land and it occurs in the day time 3. Ans (c) Explanation: • Days are hot, and during the hot season, noon temperatures of over 100°F. are quite frequent. When night falls the clear sky which promotes intense heating during the day also causes rapid radiation in the night. Temperatures drop to well below 50°F. and night frosts are not uncommon at this time of the year. This extreme diurnal range of temperature is another characteristic feature of the Sudan type of climate. • The savanna, particularly in Africa, is the home of wild animals. It is known as the ‘big game country. • The leaf and grass-eating animals include the zebra, antelope, giraffe, deer, gazelle, elephant and okapi. • Many are well camouflaged species and their presence amongst the tall greenish-brown grass cannot be easily detected. The giraffe with such a long neck can locate its enemies a great distance away, while the elephant is so huge and strong that few animals will venture to come near it. It is well equipped will tusks and trunk for defence. • The carnivorous animals like the lion, tiger, leopard, hyaena, panther, jaguar, jackal, lynx and puma have powerful jaws and teeth for attacking other animals. 4. Ans (b) Explanation: Rivers of Tamilnadu • The Thamirabarani River (Porunai) is a perennial river that originates from the famous Agastyarkoodam peak of Pothigai hills of the Western Ghats, above Papanasam in the Ambasamudram taluk. -

Western Ghats & Sri Lanka Biodiversity Hotspot
Ecosystem Profile WESTERN GHATS & SRI LANKA BIODIVERSITY HOTSPOT WESTERN GHATS REGION FINAL VERSION MAY 2007 Prepared by: Kamal S. Bawa, Arundhati Das and Jagdish Krishnaswamy (Ashoka Trust for Research in Ecology & the Environment - ATREE) K. Ullas Karanth, N. Samba Kumar and Madhu Rao (Wildlife Conservation Society) in collaboration with: Praveen Bhargav, Wildlife First K.N. Ganeshaiah, University of Agricultural Sciences Srinivas V., Foundation for Ecological Research, Advocacy and Learning incorporating contributions from: Narayani Barve, ATREE Sham Davande, ATREE Balanchandra Hegde, Sahyadri Wildlife and Forest Conservation Trust N.M. Ishwar, Wildlife Institute of India Zafar-ul Islam, Indian Bird Conservation Network Niren Jain, Kudremukh Wildlife Foundation Jayant Kulkarni, Envirosearch S. Lele, Centre for Interdisciplinary Studies in Environment & Development M.D. Madhusudan, Nature Conservation Foundation Nandita Mahadev, University of Agricultural Sciences Kiran M.C., ATREE Prachi Mehta, Envirosearch Divya Mudappa, Nature Conservation Foundation Seema Purshothaman, ATREE Roopali Raghavan, ATREE T. R. Shankar Raman, Nature Conservation Foundation Sharmishta Sarkar, ATREE Mohammed Irfan Ullah, ATREE and with the technical support of: Conservation International-Center for Applied Biodiversity Science Assisted by the following experts and contributors: Rauf Ali Gladwin Joseph Uma Shaanker Rene Borges R. Kannan B. Siddharthan Jake Brunner Ajith Kumar C.S. Silori ii Milind Bunyan M.S.R. Murthy Mewa Singh Ravi Chellam Venkat Narayana H. Sudarshan B.A. Daniel T.S. Nayar R. Sukumar Ranjit Daniels Rohan Pethiyagoda R. Vasudeva Soubadra Devy Narendra Prasad K. Vasudevan P. Dharma Rajan M.K. Prasad Muthu Velautham P.S. Easa Asad Rahmani Arun Venkatraman Madhav Gadgil S.N. Rai Siddharth Yadav T. Ganesh Pratim Roy Santosh George P.S. -

Detailed Species Accounts from The
Threatened Birds of Asia: The BirdLife International Red Data Book Editors N. J. COLLAR (Editor-in-chief), A. V. ANDREEV, S. CHAN, M. J. CROSBY, S. SUBRAMANYA and J. A. TOBIAS Maps by RUDYANTO and M. J. CROSBY Principal compilers and data contributors ■ BANGLADESH P. Thompson ■ BHUTAN R. Pradhan; C. Inskipp, T. Inskipp ■ CAMBODIA Sun Hean; C. M. Poole ■ CHINA ■ MAINLAND CHINA Zheng Guangmei; Ding Changqing, Gao Wei, Gao Yuren, Li Fulai, Liu Naifa, Ma Zhijun, the late Tan Yaokuang, Wang Qishan, Xu Weishu, Yang Lan, Yu Zhiwei, Zhang Zhengwang. ■ HONG KONG Hong Kong Bird Watching Society (BirdLife Affiliate); H. F. Cheung; F. N. Y. Lock, C. K. W. Ma, Y. T. Yu. ■ TAIWAN Wild Bird Federation of Taiwan (BirdLife Partner); L. Liu Severinghaus; Chang Chin-lung, Chiang Ming-liang, Fang Woei-horng, Ho Yi-hsian, Hwang Kwang-yin, Lin Wei-yuan, Lin Wen-horn, Lo Hung-ren, Sha Chian-chung, Yau Cheng-teh. ■ INDIA Bombay Natural History Society (BirdLife Partner Designate) and Sálim Ali Centre for Ornithology and Natural History; L. Vijayan and V. S. Vijayan; S. Balachandran, R. Bhargava, P. C. Bhattacharjee, S. Bhupathy, A. Chaudhury, P. Gole, S. A. Hussain, R. Kaul, U. Lachungpa, R. Naroji, S. Pandey, A. Pittie, V. Prakash, A. Rahmani, P. Saikia, R. Sankaran, P. Singh, R. Sugathan, Zafar-ul Islam ■ INDONESIA BirdLife International Indonesia Country Programme; Ria Saryanthi; D. Agista, S. van Balen, Y. Cahyadin, R. F. A. Grimmett, F. R. Lambert, M. Poulsen, Rudyanto, I. Setiawan, C. Trainor ■ JAPAN Wild Bird Society of Japan (BirdLife Partner); Y. Fujimaki; Y. Kanai, H. -
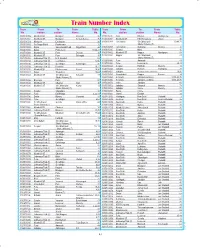
Train Number Index Train from to Train Table Train from to Train Table No
Train Number Index Train From To Train Table Train From To Train Table No. station station Name No. No. station station Name No. 10103/10104 Mumbai CST Madgaon Mandovi 26 11271/11272 Itarsi Bhopal Vindhyachal 72 10111/10112 Mumbai CST Madgaon Konkan Kanya 26 11301/11302 Mumbai CST KSR Bengaluru Udyan 10 10215/10216 Madgaon Ernakulam 26 11303/11304 Hyderabad Sri Chhatrapati 19,49,71 11001/11002 Sai Nagar Shirdi Pandharpur 10 Shahu Maharaj (T) 11003/11004 Dadar Sawantwadi Road Rajya Rani 26 11307/11308 Hyderabad Gulbarga Intercity 10 11005/11006 Dadar Puducherry 19,20,21 11309/11310 Solapur Miraj 10 11007/11008 Mumbai CST Pune Deccan 19 11401/11402 Mumbai CST Nagpur Nandigram 53 11009/11010 Mumbai CST Pune Sinhagad 19 11403/11404 Nagpur Sri Chhatrapati 44 11011/11012 Lokmanya Tilak (T) H.S. Nanded 53 Shahu Maharaj (T) 11013/11014 Lokmanya Tilak (T) Coimbatore 10,86 11405/11406 Pune Amravati 44 11015/11016 Lokmanya Tilak (T) Gorakhpur Kushinagar 35 11407/11408 Pune Lucknow Jn 35,44 11017/11018 Lokmanya Tilak (T) Karaikkal 10,21 11423/11424 Solapur Hubballi Intercity 19 11019/11020 Mumbai CST Bhubaneswar Konark 10,30 11447/11448 Jabalpur Howrah Shaktipunj 73 11021/11022 Dadar Tirunelveli 19,69 11449/11450 Jabalpur Shri Mata Vaishno Devi Katra 16,56 11023/11024 Mumbai CST Sri Chhatrapati Sahyadri 19 11453/11454 Ahmedabad Nagpur Prerana 34 Shahu Maharaj (T) 11463/11464 Somnath Jabalpur(via Itarsi) 33,34,43,76 11025/11026 Bhusaval Pune 91 11465/11466 Somnath Jabalpur (via Bina) 33,34,43,76 11027/11028 Mumbai CST Chennai Mail 10 11471/11472 Indore -

A Study on Ancient Artifacts Around Badami Hill and Their Correlation with the Natural Rock Arch Of
A Study on Ancient Artifacts Around Badami Hill and Their Correlation with the Natural Rock Arch of Sidlaphadi SHORT REPORT PRADIPTA BANERJEE MAYUR BAJAJ *Author affiliations can be found in the back matter of this article ABSTRACT CORRESPONDING AUTHOR: Pradipta Banerjee The Badami hill of Bagalkot district, Karnataka, India, houses a large rock arch termed Dayananda Sagar University, IN “Sidlaphadi” that was used as a shelter by primitive man. The hill was searched to [email protected] find any prehistoric artifact that would act as a directional marker towards the arch. An interesting structure was noted in one of the natural caves near the southwestern part of the hill at 15°55’06”N latitude and 75°41’02”E longitude. A miniature replica TO CITE THIS ARTICLE: of the arch was carved onto the floor of the cave. The axis of the miniature bridge Banerjee, P and Bajaj, M. 2021. made an angle of 28.5 ± 1.5° with the 75°41’02”E longitude. The axis, upon extension A Study on Ancient Artifacts eastwards at the defining angle reaches the northern slope of Sidlaphadi. The authors Around Badami Hill and Their also located a megalithic stone arrangement in the northern part of the hill that had Correlation with the Natural a pointed capstone and was thought to be oriented towards the rock arch. The stone Rock Arch of Sidlaphadi. Ancient Asia, 12: 9, pp. 1–9. arrangement was in the same latitude as that of the Sidlaphadi site, but the capstone DOI: https://doi.org/10.5334/ ° ° was oriented 22 E towards the winter solstice sunrise at 112.5 azimuth. -

POONDI – 613503, THANJAVUR DISTRICT STAFF PROFILE As On
A.V.V.M. SRI PUSHPAM COLLEGE (AUTONOMOUS), POONDI – 613503, THANJAVUR DISTRICT STAFF PROFILE as on : 09 .01.2019 1. Name of the Staff : Dr. M. AYYANAR 2. Designation : ASSISTANT PROFESSOR 3. Academic Qualification : M.Sc., M.Phil., Ph.D. Course UG PG M.PHIL. PH.D. Year 1999 2001 2002 2008 Ayya Nadar Janaki Ayya Nadar Janaki Ammal Ayya Nadar Janaki Ammal Loyola College, College & Ammal College, College, Sivakasi & College, Sivakasi & Madurai Chennai & University Sivakasi & Madurai Madurai Kamaraj University Kamaraj University University of Kamaraj University Madras 4. Date of Birth & Age : 08.06.1979, 39 years & 06 months D M Y 5. Date of Appointment : Self – Finance : 11 03 2009 FIP : NIL Aided : 27 11 2013 6. Total Service : 09 years & 09 months 7. Teaching Experience in 09 years 08 years 05 years completed years : UG & PG & M.Phil. & 09 months 09 months 01 month 8. Residential Address : Flat No. S-2; Karunyaa Residency No. 124/37, Gautham Nagar Nagai Road, Thanjavur – 613 001 Tamil Nadu, India Mobile Number : +91 99403 76005 E-Mail Address : [email protected] 9. No. of Orientation / Refresher : 21 - Attached as Annexure – I Courses and Training Programmes attended 10. Whether FDP availed, if yes, : No Annexure – II furnish details 11. No. of Seminars attended : 28 - Attached as Annexure – III 1 12. No. of Papers Presented : 32 - Attached as Annexure – IV 13. No. of Papers Published : 52 - Attached as Annexure – V 14. No. of Books Published : 08 (Book Chapters) attached as Annexure – VI 15. No. of Guest Lectures delivered : 04 attached as Annexure – VII in other institutions 16. -

RR 556-2019.Pdf
KFRI Research Report No. 556 ISSN: 0970-8103 ECOLOGY AND CONSERVATION GENETICS OF ATUNA INDICA AND HYDNOCARPUS LONGIPEDUNCULATUS - TWO RARE AND ENDEMIC TREES IN THE KERALA PART OF THE WESTERN GHATS P. A. Jose Suma Arundev KSCSTE- Kerala Forest Research Institute Peechi-680 653, Kerala, India (An Institution under Kerala State Council for Science, Technology& Environment) March 2019 PROJECT PARTICULARS 1. Title of the project : Ecology and conservation genetics of Atuna indica and Hydnocarpus longipedunculatus - two rare and endemic trees in the Kerala part of Western Ghats 2. Department/Organization : Kerala Forest Research Institute, Peechi. implementing the project 3. Special Area of study : i. Population survey and Mapping ii. Population structure iii. Population dynamics (Vegetative and Reproductive dynamics) iv. Climatic and edaphic factors analysis in situ v. Population genetics (Through DNA markers) vi. Assessment of species rarity and recommendation on management strategies 4. 1. Name of the principal : Dr. P.A. Jose Investigator Principal Scientist, Tree Physiology Department Sustainable Forest Management Division 2. Name of Associate Investigator : Dr. Suma Arundev Senior Scientist, Forest Genetics and Tree Breeding Department, Forest Genetics and Biotechnology Division. 3. Name of Research : 1. Mr. Jithin, K.V., Project Fellow Personnel’s (18-08 -2015 to 18.08.2016) 2. Mr. Subin, K. Project Fellow (28-08-2016 to 15.08.2018) 3. Mr. Subin, K., Project Assistant ( 22-07-2015 to 26-09-2017) 4. Mr.Vivek, A.S., Project Assistant (24-10-2016 to 03-11-2017) 5. Mr. Binoy, N.M., Project Assistant (07-12-2017 to 13-03-2018) 5 . Name of the Funding : Plan Grant of Kerala Forest Agency Research institute, Peechi, Thrissur 6 . -

Governance Innovation Networks for Sustainable Tuna Governance Innovation
Tuna Sustainable for Networks Innovation Governance Governance Innovation Networks for Sustainable Tuna Alice M.M. Miller 2014 Alice M.M. Miller Governance Innovation Networks for Sustainable Tuna Alice M.M. Miller Thesis committee Promotor Prof. Dr A.P.J. Mol Professor of Environmental Policy Wageningen University Co-promotor Dr S.R. Bush Associate professor, Environmental Policy Group Wageningen University Other members Dr L. Campling, Queen Mary’s of University, London, UK Dr P.J. Jones, University College London, UK Prof. Dr A.D. Rijnsdorp, Wageningen University Prof. Dr C.J.A.M. Termeer, Wageningen University This research was conducted under the auspices of the Wageningen School of Social Sciences Governance Innovation Networks for Sustainable Tuna Alice M.M. Miller Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by authority of the Rector Macnificus Prof. Dr M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Thursday 2 October 2014 at 11 a.m. in the Aula. Alice M.M. Miller Governance Innovation Networks for Sustainable Tuna, 194 pages. PhD thesis, Wageningen University, Wageningen, NL (2014) With references, with summaries in Dutch and English ISBN 978-94-6257-025-2 Acknowledgements The foundation of this thesis is networks. Through over four years of research in two different universities, I developed my understanding of networks in the context of tuna governance but also on a personal level. It is through my network of colleagues, friends and family that I have been able to work on and finish this thesis and even to enjoy the process (mostly!) I would therefore like to take this opportunity to thank all those people who have offered help and support to me over the years in their own individual ways. -

Tuna Supply Chains and Regulatory Frameworks in Two Pacific Island
Ver.July28 TunaSupplyChainsandRegulatory FrameworksinTwoPacificIsland Countries MikeA.McCoy July28,2014 GILLETT, PRESTON AND ASSOCIATES INC. 2 SUMMARY OF MAJOR FINDINGS Tuna purse seining in x Largest purse seine catch among all PICs Papua New Guinea x Conducted by a mix of domestic and foreign vessels Papua New Guinea fleet x 234 purse seine vessels licensed in 2012, 90 percent in 50- structure and productivity 80 meter length class x Total 2012 purse seine catch in PNG waters 518,349 tons, of which 9 percent by PNG flag, 22 percent by locally-based foreign flag, 69 percent by distant water vessels x total reported purse seine catch in PNG waters has decreased in each of the past three years Commercial entities x Two categories: PNG-registered, foreign owned, 9 involved in fishing companies activities x Foreign with licensed vessels under bilateral agreements, 9 companies or associations, excluding US tuna treaty Key features of fishing x For vessels associated with processing in PNG, some catch operations sold locally, significant amounts exported, some processed locally x Extensive use of anchored FADs x Transshipment common for domestic vessels x Supply patterns differ among 3 top producers Processing of skipjack x Estimated less than one-third of total catch landed in PNG caught and landed in PNG for processing x 5 canning or loining facilities, 3 owned by Philippine firms, one by Taiwan, one Malaysia x Processors invest to gain access to resources and for preferential access to EU market x Significant challenges to operating processing -

Johncy Vanam'
'SHANTHISTHAL' (JOHNCYVANAM) 2013-2018 In Collaboration with Kerala State Biodiversity Board and with the technical support of Department of Botany and M. S. Swaminathan Research Foundation Payyanur college Biodiversity Club established a conservation garden ('Shanthisthal') of Rare Endemic and Threatened flowering plants (RET plants) at Payyanur college campus in 1 acre area. Two hundred and thirty seven seedlings of 71 species of Rare Endemic and Threatened (RET) flowering plants (Angiosperms) of the Western Ghats coming in 29 families have been planted and conserved in the garden. Dr. P.S. Easa, former Director of Kerala Forest Research Institute formally inaugurated the garden as 'Johncyvanam' on 21st October, 2016 (in the name of Prof. Johncy Jacob, former professor of Department of Zoology, Payyanur College) and dedicated to the founders and retired teachers of Payyanur College. More than 65% of these species are coming under various threat categories of IUCN (Nayar, 1997). Among these Vatica chinensis, Poeciloneuron pauciflorum, Nothopegia heyneana and Aglaia malabarica are 'Critically Endangered' (CR) tree species and Syzygium occidentalis, Kunstleria keralensis, Saraca asoca, Myristica malabarica and Palaquium bourdillonii listed as 'Vulnerable' (VU). Nine tree species like Dipterocarpus indicus, Hopea parviflora, and Syzygium stocksii are coming under the category “Endangered” (E). Humboldtia vahliana Vepris bilocularis, Phaeanthus malabaricus and Actinodaphne malabarica are coming under the 'Rare' (R) category of IUCN Red Data Book. Thirteen plants are coming under the IUCN category of 'Locally Rare'. Some of them are Baccaurea courtallensis, Cullenia exarillata, Diospyros pruriens, Flacourtia montana, Otonephelium stipulaceum, Artocarpus hirsutus, and Cinnamomum sulphuratum. Gluta travancorica, and Sageraea laurina are coming under the category of 'Lower Risk' or 'Near Threatened'. -

Chapter 6 ENUMERATION
Chapter 6 ENUMERATION . ENUMERATION The spermatophytic plants with their accepted names as per The Plant List [http://www.theplantlist.org/ ], through proper taxonomic treatments of recorded species and infra-specific taxa, collected from Gorumara National Park has been arranged in compliance with the presently accepted APG-III (Chase & Reveal, 2009) system of classification. Further, for better convenience the presentation of each species in the enumeration the genera and species under the families are arranged in alphabetical order. In case of Gymnosperms, four families with their genera and species also arranged in alphabetical order. The following sequence of enumeration is taken into consideration while enumerating each identified plants. (a) Accepted name, (b) Basionym if any, (c) Synonyms if any, (d) Homonym if any, (e) Vernacular name if any, (f) Description, (g) Flowering and fruiting periods, (h) Specimen cited, (i) Local distribution, and (j) General distribution. Each individual taxon is being treated here with the protologue at first along with the author citation and then referring the available important references for overall and/or adjacent floras and taxonomic treatments. Mentioned below is the list of important books, selected scientific journals, papers, newsletters and periodicals those have been referred during the citation of references. Chronicles of literature of reference: Names of the important books referred: Beng. Pl. : Bengal Plants En. Fl .Pl. Nepal : An Enumeration of the Flowering Plants of Nepal Fasc.Fl.India : Fascicles of Flora of India Fl.Brit.India : The Flora of British India Fl.Bhutan : Flora of Bhutan Fl.E.Him. : Flora of Eastern Himalaya Fl.India : Flora of India Fl Indi. -

10Th-Std-Social-2Nd-Volume-Book
General Studies Prepared By www.winmeen.com 10th Social 2nd Volume Book Back Questions History Unit 6 - Early Revolts against British Rule in Tamil Nadu Unit 7 - Anti-Colonial Movements and the Birth of Nationalism Unit 8 - Nationalism: Gandhian Phase Unit 9 - Freedom Struggle in Tamil Nadu Unit 10 - Social Transformation in Tamil Nadu Geography Unit 6 - Physical Geography of Tamil Nadu Unit 7 - Human Geography of Tamil Nadu Civics Unit 4 - India’s Foreign Policy Unit 5 - India’s International Relations Economics Unit 3 - Food Security and Nutrition Unit 4 - Government and Taxes Unit 5 - Industrial Clusters in Tamil Nadu Learning Leads To Ruling Page 1 of 103 General Studies Prepared By www.winmeen.com HISTORY Unit - 6 Early revolts against British rule in Tamil Nadu Choose the Correct Answer: 1. Who was the first Palayakkarars to resist the East India Company’s policy of territorial aggrandizement? (a) Marudhu brothers (b) Puli Thevar (c) Velunachiyar (d) Veerapandya Kattabomman 2. Who had borrowed money from the East India Company to meet the expenses he had incurred during the Carnatic wars? (a) Velunachiyar (b) Puli Thevar (c) Nawab to Arcot (d) Raja of Travancore 3. Who had established close relationship with the three agents of Chanda Sahib? (a) Velunachiyar (b) Kattabomman (c) Puli Thevar (d) Oomai Thurai 4. Where was Sivasubramanianar executed? (a) Kayathar (b) Nagalapuram (c) Virupachi (d) Panchalamkurichi 5. Who issued the Tiruchirappalli proclamation of Independence? (a) Marudhu brothers (b) Puli Thevar (c) Veerapandya Kattabomman (d) Gopala Nayak 6. When did the Vellore Revolt breakout? (a) 24 May 1805 (b) 10 July 1805 (c) 10 July 1806 (d) 10 September 1806 7.