Inhibition Mechanism of an Anti-CRISPR Suppressor Acriia4 Targeting Spycas9
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2012 Annual Report
Memorial Sloan-Kettering Cancer Center 2012 ANNUAL REPORT A SHARED VISION A SINGULAR MISSION Nurse practitioner Naomi Cazeau, of the Adult Bone Marrow Transplant Service. PING CHI PHYSICIAN-SCIENTIST 10 STEPHEN SOLOMON ALEXANDER RUDENSKY INTERVENTIONAL IMMUNOLOGIST RADIOLOGIST 16 12 VIVIANE TABAR The clinicians and scientists of NEUROSURGEON Memorial Sloan-Kettering share a vision and 18 a singular mission — to conquer cancer. STEPHEN LONG STRUCTURAL BIOLOGIST They are experts united against a 20 SIMON POWELL complex disease. Each type of cancer R ADIATION ONCOLOGIST 24 ETHEL LAW is different, each tumor is unique. Set free NURSE PRACTITIONER in surroundings that invite the sharing of 26 ideas and resources, they attack the CHRISTINA LESLIE complexity of cancer from every angle COMPUTATIONAL BIOLOGIST and every discipline. 34 SCOTT ARMSTRONG PEDIATRIC ONCOLOGIST 30 TO JORGE REIS-FILHO EXPERIMENTAL PATHOLOGIST CONQUER 38 CANCER 04 Letter from the Chairman and the President A complete version of this report — 42 Statistical Profile which includes lists of our donors, 44 Financial Summary doctors, and scientists — 46 Boards of Overseers and Managers is available on our website at 49 The Campaign for Memorial Sloan-Kettering www.mskcc.org/annualreport. 4 5 Letter from the Chairman In 2012 the leadership of Memorial Sloan-Kettering endorsed Douglas A. Warner III These programmatic investments require leadership and and the President a $2.2 billion investment in a clinical expansion that will set vision. Our new Physician-in-Chief, José Baselga, joined the stage for a changing care paradigm into the next decade us on January 1, 2013. An internationally recognized and beyond. -

2011 Annual Report
Memorial Sloan-Kettering Cancer Center 2011 Annual Report 10 steps closer 10 steps closer Letter from the Chairman and the President 1 1 | First effective treatments for advanced melanoma 5 2 Genomic analysis offers clues to most common | type of ovarian cancer 7 3 Breast cancer surgery: practice-changing | findings for some patients 9 4 New drugs offer survival benefit for men | with metastatic prostate cancer 11 5 | Insights into DNA damage and repair 13 6 Novel stem cell technique shows promise | in treating disease 15 7 Combination therapy may prevent spread | of nasopharyngeal tumors 17 8 Algorithm can predict shape of proteins, | speeding basic cancer research 19 9 Two of 2011’s top five advances in cancer | research led by MSKCC physician-scientists 21 10 | The Josie Robertson Surgery Center 23 The Campaign for Memorial Sloan-Kettering 25 Statistical Profile 27 Financial Summary 29 Boards of Overseers and Managers 31 www.mskcc.org/annualreport Letter from the Chairman and the President The year 2011 was a strong one at Memorial Sloan-Kettering. We continued to lead across “Our success as an institution is due in the spectrum of patient care, research, and training, and laid the groundwork for important progress in the years ahead. great measure to our remarkable staff… We want to begin by saying that our success as an institution is due in great measure to our remarkable staff. On a daily basis, we are inspired by their dedication and compassion, and We are inspired by their dedication Douglas A. Warner III are grateful for the work they do in the service of our patients and our mission. -

Dual Recognition of H3k4me3 and H3k27me3 by a Plant Histone Reader SHL
ARTICLE DOI: 10.1038/s41467-018-04836-y OPEN Dual recognition of H3K4me3 and H3K27me3 by a plant histone reader SHL Shuiming Qian1,2, Xinchen Lv3,4, Ray N. Scheid1,2,LiLu1,2, Zhenlin Yang3,4, Wei Chen3, Rui Liu3, Melissa D. Boersma2, John M. Denu2,5,6, Xuehua Zhong 1,2 & Jiamu Du 3 The ability of a cell to dynamically switch its chromatin between different functional states constitutes a key mechanism regulating gene expression. Histone mark “readers” display 1234567890():,; distinct binding specificity to different histone modifications and play critical roles in reg- ulating chromatin states. Here, we show a plant-specific histone reader SHORT LIFE (SHL) capable of recognizing both H3K27me3 and H3K4me3 via its bromo-adjacent homology (BAH) and plant homeodomain (PHD) domains, respectively. Detailed biochemical and structural studies suggest a binding mechanism that is mutually exclusive for either H3K4me3 or H3K27me3. Furthermore, we show a genome-wide co-localization of SHL with H3K27me3 and H3K4me3, and that BAH-H3K27me3 and PHD-H3K4me3 interactions are important for SHL-mediated floral repression. Together, our study establishes BAH-PHD cassette as a dual histone methyl-lysine binding module that is distinct from others in recognizing both active and repressive histone marks. 1 Laboratory of Genetics, University of Wisconsin-Madison, Madison, WI 53706, USA. 2 Wisconsin Institute for Discovery, University of Wisconsin-Madison, Madison, WI 53706, USA. 3 National Key Laboratory of Plant Molecular Genetics, CAS Center for Excellence in Molecular Plant Sciences, Shanghai Center for Plant Stress Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 201602, China. -
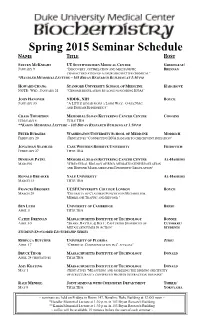
Spring 2015 BCH Sem Sched
Spring 2015 Seminar Schedule Name Title Host STEVEN MCKNIGHT UT SOUTHWESTERN MEDICAL CENTER GREENLEAF/ JANUARY 9 “DISCOVERY, OPTIMIZATION AND MECHANISTIC BRENNAN CHARACTERIZATION OF A NEUROPROTECTIVE CHEMICAL” *HANDLER MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM HOWARD CHANG STANFORD UNIVERSITY SCHOOL OF MEDICINE HARGROVE NOTE: WED., JANUARY 21 “GENOME REGULATION BY LONG NONCODING RNAS” JOHN HANOVER NIDDK, NIH BOYCE JANUARY 30 “A LITTLE SUGAR GOES A LONG WAY: O-GLCNAC AND DISEASE EPIGENETICS” CRAIG THOMPSON MEMORIAL SLOAN KETTERING CANCER CENTER COGGINS FEBRUARY 6 TITLE TBA **KAMIN MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM PETER BURGERS WASHINGTON UNIVERSITY SCHOOL OF MEDICINE MODRICH FEBRUARY 20 (TENTATIVE) “CONNECTING DNA DAMAGE TO CHECKPOINT INITIATION” JONATHAN STAMLER CASE WESTERN RESERVE UNIVERSITY FRIDOVICH FEBRUARY 27 TITLE TBA DINSHAW PATEL MEMORIAL SLOAN KETTERING CANCER CENTER AL-HASHIMI MARCH 6 "STRUCTURAL BIOLOGY OF RNA-MEDIATED GENE REGULATION AND HISTONE MARK-MEDIATED EPIGENETIC REGULATION" RONALD BREAKER YALE UNIVERSITY AL-HASHIMI MARCH 13 TITLE TBA FRANCES BRODSKY UCSF/UNIVERSITY COLLEGE LONDON BOYCE MARCH 20 "DIVERSITY OF CLATHRIN FUNCTION IN METABOLISM, MEMBRANE TRAFFIC AND BEYOND " BEN LUISI UNIVERSITY OF CAMBRIDGE BEESE APRIL 3 TITLE TBA CATHY DRENNAN MASSACHUSETTS INSTITUTE OF TECHNOLOGY BONNIE APRIL 10 "SHAKE, RATTLE, & ROLL: CAPTURING SNAPSHOTS OF CUTHBERT/ METALLOENZYMES IN ACTION" STUDENTS STUDENT-SPONSORED LECTURESHIP SERIES REBECCA BUTCHER UNIVERSITY OF FLORIDA ZHOU APRIL 17 “CHEMICAL COMMUNICATION IN C. ELEGANS” BRUCE TIDOR MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD APRIL 24 (TENTATIVE) TITLE TBA AMY KEATING MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD MAY 1 (TENTATIVE) "MEASURING AND MODELING THE BINDING SPECIFICITY OF STRUCTURALLY CONSERVED PROTEIN INTERACTION DOMAINS" RALF MENDEL JOINT SEMINAR WITH CHEMISTRY DEPARTMENT THIELE/ MAY 8 TITLE TBA YOKOYAMA ~ seminars are held on Fridays in Room 147, Nanaline Duke Building at 12:OO noon ~ *Handler Memorial Lecture at 1:30 p.m. -

Describing the Elephant the Three-Dimensional Structures of Rnas Are Notoriously Difficult to Determine
SCOTT BASKERVILLE AND ANDREW D. ELLINGTON RNA STRUCTURE Describing the elephant The three-dimensional structures of RNAs are notoriously difficult to determine. Functional comparisons of variant molecules and cross-linking experiments are providing new information for structural modeling. The old fable of the blind men trying to describe the in vitro selection is the Rev-binding element (RBE) of shape of an elephant seems aptly to describe the present HIV-1. RBE is a 30-base sequence of the HIV-1 genome state of RNA structural analysis. Until recently, the crys- that can fold into a 'stem-internal-loop-stem' secondary tal structure of only one large RNA, tRNA, was known, structure and that interacts with the Rev protein, and and other non-crystallographic approaches to RNA regulates the splicing and transport of viral mRNAs. structure determination had not been particularly fruit- From a population of partially randomized sequences, ful. Low-resolution electron micrographs are at best able Bartel and Szostak [3] selected RBE variants that could to identify the global structure of the ribosome, but can- bind the Rev protein, whereas Giver et al. [4] selected not provide three-dimensional structural details. The binding variants from a population of completely random nuclear magnetic resonance (NMR) spectra of most sequences. These experiments revealed numerous se- RNAs are so complex that this method of structure quence co-variations that were used by Leclerc et al. [5] determination is limited to molecules of molecular mass 10 000 daltons or less. But new methods now provide at least partial insights into RNA structures. -

Research Report 2009 Max Planck Institute for Molecular Genetics, Berlin Imprint | Research Report 2009
Research Report 2009 Max Planck Institute for Molecular Genetics, Berlin Imprint | Research Report 2009 Published by the Max Planck Institute for Molecular Genetics (MPIMG), Berlin, Germany, December 2009 Editorial Board: B.G. Herrmann, H. Lehrach, H.-H. Ropers, M. Vingron Conception & coordination: Patricia Marquardt Photography: Katrin Ullrich, MPIMG; David Ausserhofer Scientific Illustrations: MPIMG Production: Thomas Didier, Meta Data Contact: Max Planck Institute for Molecular Genetics Ihnestr. 63 – 73 14195 Berlin Germany Phone: +49 (0)30 8413-0 Fax: +49 (0)30 8413-1207 Email: [email protected] For further information about the MPIMG, please visit http://www.molgen.mpg.de MPI for Molecular Genetics Research Report 2009 Research Report 2009 1 Max Planck Institute for Molecular Genetics Berlin, December 2009 The Max Planck Institute for Molecular Genetics 2 MPI for Molecular Genetics Research Report 2009 Table of contents Organisational structure . 6 The Max Planck Institute for Molecular Genetics . 7 Mission . 7 Development of the Institute. 7 Research Concept . 8 Department of Developmental Genetics (Bernhard Herrmann) . 9 Transmission ratio distortion (H. Bauer) . 13 Regulatory Networks of Mesoderm Formation & Somitogenesis (B. Herrmann) . 17 Signal Transduction in Embryogenesis and Tumour Progression (M. Morkel) . 22 Organogenesis (H. Schrewe) . 26 General information about the whole Department . 29 Department of Vertebrate Genomics (Hans Lehrach) . 33 Molecular Embryology and Aging (J. Adjaye) . 40 Neuropsychiatric Genetics (L. Bertram) . 46 Automation (A. Dahl, W. Nietfeld, H. Seitz) . 49 Nucleic Acid-based Technologies (J. Glökler) . 55 Bioinformatics (R. Herwig) . 60 Comparative and Functional Genomics (H. Himmelbauer) . .65 Genetic Variation, Haplotypes & Genetics of Complex Diseases (M. Hoehe) . 69 3 in vitro Ligand Screening (Z. -

Riding in Silence: a Little Snowboarding, a Lot of Small Rnas Stefan L Ameres*, Ryuya Fukunaga*
Ameres and Fukunaga Silence 2010, 1:8 http://www.silencejournal.com/content/1/1/8 REVIEW Open Access Riding in silence: a little snowboarding, a lot of small RNAs Stefan L Ameres*, Ryuya Fukunaga* Abstract The recent symposium, RNA silencing: Mechanism, Biology and Applications, organized by Phillip D. Zamore (University of Massachusetts Medical School) and Beverly Davidson (University of Iowa), and held in Keystone, Colorado, brought together scientists working on diverse aspects of RNA silencing, a field that comprises a multitude of gene regulatory pathways guided by microRNAs, small interfering RNAs and PIWI-interacting RNAs. Review side of the stem. To explain these data, Kim proposed From 14 to 19 January 2010, small RNAs once again an alternative model to the PAZ-anchored molecular attracted the attention of more than 500 attendees to ruler model that emerged from the structure of Giardia symposium on RNA silencing: Mechanism, Biology and Dicer [3]. She suggested that Dicer proteins in higher Applications, held in Keystone, Colorado. In the midst eukaryotes measure mainly from the 5’ end of a pre- of the breathtaking panorama of the Rocky Mountain miRNA, rather than from its 3’ end. summits, exciting and rapidly evolving science mixed Jennifer Doudna (University of California, Berkeley) with superb riding and skiing created a stimulating described the first structural insights into human pre- atmosphere for discussions on the mechanisms, biology miRNA processing and loading by electron microscopy and applications of RNA silencing. [4]. She presented biochemical cross-linking studies employing a recombinant human Dicer-TRBP (trans acti- Biogenesis of small RNAs vation response RNA binding protein) complex. -

Download the Annual Report 2011
Centre for Genomic Regulation Annual Report 2011 © Copyright 2012 Produced by: Department of Communication & Public Relations Centre for Genomic Regulation (CRG) Dr. Aiguader, 88 08003 Barcelona, Spain www.crg.eu Texts and graphics: CRG scientists, CRG members of the management team, Cristina Sáez, Anabel Herrera, Department of Communication & Public Relations Graphic Design: Genoma ArtStudio SCP (www.genoma-artstudio.com) Photography: Ivan Martí and CRG scientists Cover photography: Luis Bejarano Printing: Escardó, S.L. Legal deposit: B-17275-2012 CONTENTS Message from the Former Director 7 117 > Cell and Developmental Biology 117 Intracellular Compartmentation Foreword 9 121 Microtubule Function and Cell Division 125 Sensory Cell Biology and Organogenesis A Look Back at the Year 10 129 Cytokinesis and Chromosome Segregation > Research 12 133 Biomechanics of Morphogenesis > Core Facilities 14 137 Organelle Biogenesis and Homeostasis > Advanced Training 15 140 > Systems Biology > Alumni 18 140 Systems Analysis of Development > Technology Transfer 18 144 Design of Biological Systems > Outreach 18 149 Gene Network Engineering > General Administration 20 153 Genetic Systems > Grants & Scientific and International Affairs 22 157 Sensory Systems and Behaviour 161 Comparative Analysis of Developmental Systems Scientific Highlights 26 166 > Core Facilities > Gene Regulation: Reading a gene in a minute 27 166 Ultrasequencing Unit > Differentiation and Cancer: Revealing the cell’s biological clock 29 170 Genotyping Unit > Genes and Disease: In -

2013 Annual Report
2013 Annual Report Transformations Our vision is nothing less than to revolutionize the treatment of cancer. Introduction MEMORIAL SLOAN KETTERING CANCER CENTER 2 Table of Contents 3 Transforming 04 Message from the Chairman and the President 60 Statistical Profile Biomedical 62 Financial Summary Our goal over the next decade is 64 Boards of Overseers 08 Research and Managers 65 Principal Leadership to integrate molecular and clinical 67 Facilities Update 69 The Campaign for Transforming information to develop therapies Memorial Sloan Kettering that home in on the abnormalities Cancer Center Drug Discovery 71 Donors to the Campaign for driving each patient’s disease. Memorial Sloan Kettering 20 & Development Cancer Center 86 The Society of Memorial Sloan Kettering Cancer Center At Memorial Sloan Kettering, this new era in precision cancer medicine is already becoming a reality for many of our patients. Transforming Capitalizing on our exceptionally powerful combination of clinical and scientific resources, we are delivering on the promise Precision of personalized cancer therapy and are setting the stage for transformational change, both in the immediate future and for 30 Medicine years to come. Join us now on a journey that will span our institution. It begins with the outstanding biomedical research that informs novel Transforming early-stage drug discovery and development, takes us through paradigm-breaking precision medicine and robust clinical research, Clinical and concludes with innovative new approaches that will transform individualized -

2016-2017 Hormel Institute Annual Report
Cellular and Molecular Biology 6 Cancer Biomarkers and Drug Resistance 10 Molecular Therapeutics & Cancer Health Disparity 12 Membrane Biochemistry 16 Structural Biology 18 Nutrition and Metabolism 20 Today’s RESEARCH, Cell Death and Cancer Genetics 22 Cancer Cell Biology and Translational Research 26 Tomorrow’s CURES Cellular Dynamics 28 Tumor Microenvironment and Metastasis 32 Cryo-EM and Molecular Cell Biology 34 Cancer Epigenetics & Experimental Therapeutics 36 Stem Cells and Cancer 40 Cell Signaling and Tumorigenesis 42 Molecular Biology and Translational Cancer Research 44 Historical facts for the special section, Then & Now, were compiled from a number of different Partners in Growth 52 sources including past Annual Reports of The Hormel Institute, historical copies of Austin Daily Herald and other historical documents from the Mower County Historical Society, the article “The University of Minnesota’s Hormel Institute: The first 66 years of a unique scientific enterprise” by 75th Anniversary "Then & Now" 58 Harald H. O. Schmid, the book In Quest of Quality: Hormel’s First 75 Years by Richard Dougherty, and the book The University of Minnesota, 1945-2000 by Stanford Lehmberg and Ann M. Pflaum. 2 The Hormel Institute The mission of The Hormel Institute is to conduct research and provide education in the biological sciences with applications in medicine and agriculture. In pursuit of this mission, and as intended by its founders, The Hormel Institute generates fundamental knowledge and disseminates it to the scientific community worldwide. It also serves as a center of technical and educational expertise for the benefit of the Austin community, the surrounding region and the State of Minnesota. -

A Little Snowboarding, a Lot of Small Rnas
University of Massachusetts Medical School eScholarship@UMMS Open Access Articles Open Access Publications by UMMS Authors 2010-03-17 Riding in silence: a little snowboarding, a lot of small RNAs Stefan L. Ameres University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Genetics Commons, and the Medicine and Health Sciences Commons Repository Citation Ameres SL, Fukunaga R. (2010). Riding in silence: a little snowboarding, a lot of small RNAs. Open Access Articles. https://doi.org/10.1186/1758-907X-1-8. Retrieved from https://escholarship.umassmed.edu/ oapubs/2231 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Articles by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. Ameres and Fukunaga Silence 2010, 1:8 http://www.silencejournal.com/content/1/1/8 REVIEW Open Access Riding in silence: a little snowboarding, a lot of small RNAs Stefan L Ameres*, Ryuya Fukunaga* Abstract The recent symposium, RNA silencing: Mechanism, Biology and Applications, organized by Phillip D. Zamore (University of Massachusetts Medical School) and Beverly Davidson (University of Iowa), and held in Keystone, Colorado, brought together scientists working on diverse aspects of RNA silencing, a field that comprises a multitude of gene regulatory pathways guided by microRNAs, small interfering RNAs and PIWI-interacting RNAs. Review side of the stem. To explain these data, Kim proposed From 14 to 19 January 2010, small RNAs once again an alternative model to the PAZ-anchored molecular attracted the attention of more than 500 attendees to ruler model that emerged from the structure of Giardia symposium on RNA silencing: Mechanism, Biology and Dicer [3]. -

A CASP-Like Evaluation of RNA Three-Dimensional Structure Prediction
Downloaded from rnajournal.cshlp.org on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press BIOINFORMATICS RNA-Puzzles: A CASP-like evaluation of RNA three-dimensional structure prediction JOSE´ ALMEIDA CRUZ,1 MARC-FRE´DE´RICK BLANCHET,2 MICHAL BONIECKI,3 JANUSZ M. BUJNICKI,3,4 SHI-JIE CHEN,5 SONG CAO,5 RHIJU DAS,6,7 FENG DING,8 NIKOLAY V. DOKHOLYAN,8 SAMUEL COULBOURN FLORES,9 LILI HUANG,10 CHRISTOPHER A. LAVENDER,11 VE´RONIQUE LISI,2 FRANCxOIS MAJOR,2 KATARZYNA MIKOLAJCZAK,3 DINSHAW J. PATEL,10 ANNA PHILIPS,3,4 TOMASZ PUTON,4 JOHN SANTALUCIA,12,13 FREDRICK SIJENYI,13 THOMAS HERMANN,14 KRISTIAN ROTHER,4 MAGDALENA ROTHER,4 ALEXANDER SERGANOV,10 MARCIN SKORUPSKI,4 TOMASZ SOLTYSINSKI,3 PARIN SRIPAKDEEVONG,6,7 IRINA TUSZYNSKA,3 KEVIN M. WEEKS,11 CHRISTINA WALDSICH,15 MICHAEL WILDAUER,15 NEOCLES B. LEONTIS,16 and ERIC WESTHOF1,17 1Architecture et Re´activite´ de l’ARN, Universite´ de Strasbourg, IBMC-CNRS, F-67084 Strasbourg, France 2Institute for Research in Immunology and Cancer (IRIC), Department of Computer Science and Operations Research, Universite´ de Montre´al, Montre´al, Que´bec H3C 3J7, Canada 3Laboratory of Bioinformatics and Protein Engineering, International Institute of Molecular and Cell Biology in Warsaw, 02-109 Warsaw, Poland 4Laboratory of Bioinformatics, Institute of Molecular Biology and Biotechnology, Faculty of Biology, Adam Mickiewicz University, 61-614 Poznan, Poland 5Department of Physics and Department of Biochemistry, University of Missouri, Columbia, Missouri 65211, USA 6Department of Biochemistry,