2011 Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BRCA1, BRCA2 and Beyond: an Update on Hereditary Cancer Keynote Speaker and Basser Global Prize Awardee: Maria Jasin, Phd
TH 7 ANNUAL SCIENTIFIC SYMPOSIUM A CME/CNE – Certified Conference BRCA1, BRCA2 and Beyond: An Update on Hereditary Cancer Keynote Speaker and Basser Global Prize Awardee: Maria Jasin, PhD TUESDAY, MAY 7, 2019 WEDNESDAY, MAY 8, 2019 Smilow Center for Translational Research Auditorium/Commons (enter through Perelman Center for Advanced Medicine) Program Overview This symposium is designed to educate researchers, scientists, and health care providers in cancer genetics by presenting cutting-edge data from renowned BRCA1/2 researchers. This symposium will provide new information on the basic biology of known cancer susceptibility genes. In addition, progress in cancer screening and prevention as well as ongoing work in targeted therapy for BRCA1/2 mutation carriers will be discussed through a series of lectures and panel discussions. Attendees will have the opportunity to network with colleagues working in the field. PROGRAM OBJECTIVES At the completion of this symposium, attendees should be able to: • Discuss new research on the basic biology of BRCA1 and BRCA2 and other homologous repair deficiency genes. • Describe current ethical, legal and social issues associated with carrying a BRCA1/2 mutation. • Review new approaches to screening and prevention of hereditary breast and ovarian cancer. • Discuss new advances in cancer treatment for BRCA1/2 mutation carriers. WHO SHOULD ATTEND Healthcare providers including medical oncology, surgical oncology, gynecology oncology, ob-gyn, genetic counselors, primary care physicians and nurses/nurse practitioners who are interested in the genetics of BRCA1/2 related cancers. KEYNOTE SPEAKER AND BASSER GLOBAL PRIZE AWARDEE Maria Jasin, PhD Memorial Sloan Kettering Cancer Center Maria Jasin, PhD, is a member of the Developmental Biology Program at Memorial Sloan Kettering Cancer Center and a professor at the Weill Graduate School of Medical Sciences at Cornell University. -

Looking at Earth: an Astronaut's Journey Induction Ceremony 2017
american academy of arts & sciences winter 2018 www.amacad.org Bulletin vol. lxxi, no. 2 Induction Ceremony 2017 Class Speakers: Jane Mayer, Ursula Burns, James P. Allison, Heather K. Gerken, and Gerald Chan Annual David M. Rubenstein Lecture Looking at Earth: An Astronaut’s Journey David M. Rubenstein and Kathryn D. Sullivan ALSO: How Are Humans Different from Other Great Apes?–Ajit Varki, Pascal Gagneux, and Fred H. Gage Advancing Higher Education in America–Monica Lozano, Robert J. Birgeneau, Bob Jacobsen, and Michael S. McPherson Redistricting and Representation–Patti B. Saris, Gary King, Jamal Greene, and Moon Duchin noteworthy Select Prizes and Andrea Bertozzi (University of James R. Downing (St. Jude Chil- Barbara Grosz (Harvard Univer- California, Los Angeles) was se- dren’s Research Hospital) was sity) is the recipient of the Life- Awards to Members lected as a 2017 Simons Investi- awarded the 2017 E. Donnall time Achievement Award of the gator by the Simons Foundation. Thomas Lecture and Prize by the Association for Computational American Society of Hematology. Linguistics. Nobel Prize in Chemistry, Clara D. Bloomfield (Ohio State 2017 University) is the recipient of the Carol Dweck (Stanford Univer- Christopher Hacon (University 2017 Robert A. Kyle Award for sity) was awarded the inaugural of Utah) was awarded the Break- Joachim Frank (Columbia Univer- Outstanding Clinician-Scientist, Yidan Prize. through Prize in Mathematics. sity) presented by the Mayo Clinic Di- vision of Hematology. Felton Earls (Harvard Univer- Naomi Halas (Rice University) sity) is the recipient of the 2018 was awarded the 2018 Julius Ed- Nobel Prize in Economic Emmanuel J. -

2012 Annual Report
Memorial Sloan-Kettering Cancer Center 2012 ANNUAL REPORT A SHARED VISION A SINGULAR MISSION Nurse practitioner Naomi Cazeau, of the Adult Bone Marrow Transplant Service. PING CHI PHYSICIAN-SCIENTIST 10 STEPHEN SOLOMON ALEXANDER RUDENSKY INTERVENTIONAL IMMUNOLOGIST RADIOLOGIST 16 12 VIVIANE TABAR The clinicians and scientists of NEUROSURGEON Memorial Sloan-Kettering share a vision and 18 a singular mission — to conquer cancer. STEPHEN LONG STRUCTURAL BIOLOGIST They are experts united against a 20 SIMON POWELL complex disease. Each type of cancer R ADIATION ONCOLOGIST 24 ETHEL LAW is different, each tumor is unique. Set free NURSE PRACTITIONER in surroundings that invite the sharing of 26 ideas and resources, they attack the CHRISTINA LESLIE complexity of cancer from every angle COMPUTATIONAL BIOLOGIST and every discipline. 34 SCOTT ARMSTRONG PEDIATRIC ONCOLOGIST 30 TO JORGE REIS-FILHO EXPERIMENTAL PATHOLOGIST CONQUER 38 CANCER 04 Letter from the Chairman and the President A complete version of this report — 42 Statistical Profile which includes lists of our donors, 44 Financial Summary doctors, and scientists — 46 Boards of Overseers and Managers is available on our website at 49 The Campaign for Memorial Sloan-Kettering www.mskcc.org/annualreport. 4 5 Letter from the Chairman In 2012 the leadership of Memorial Sloan-Kettering endorsed Douglas A. Warner III These programmatic investments require leadership and and the President a $2.2 billion investment in a clinical expansion that will set vision. Our new Physician-in-Chief, José Baselga, joined the stage for a changing care paradigm into the next decade us on January 1, 2013. An internationally recognized and beyond. -

Resubmission JBC Frescas and De Lange
Cell Biology: Binding of TPP1 Protein to TIN2 Protein Is Required for POT1a,b Protein-mediated Telomere Protection David Frescas and Titia de Lange J. Biol. Chem. 2014, 289:24180-24187. doi: 10.1074/jbc.M114.592592 originally published online July 23, 2014 Downloaded from Access the most updated version of this article at doi: 10.1074/jbc.M114.592592 http://www.jbc.org/ Find articles, minireviews, Reflections and Classics on similar topics on the JBC Affinity Sites. Alerts: • When this article is cited • When a correction for this article is posted at Rockefeller University Library on August 31, 2014 Click here to choose from all of JBC's e-mail alerts This article cites 29 references, 13 of which can be accessed free at http://www.jbc.org/content/289/35/24180.full.html#ref-list-1 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 289, NO. 35, pp. 24180–24187, August 29, 2014 © 2014 by The American Society for Biochemistry and Molecular Biology, Inc. Published in the U.S.A. Binding of TPP1 Protein to TIN2 Protein Is Required for POT1a,b Protein-mediated Telomere Protection* Received for publication, June 30, 2014, and in revised form, July 22, 2014 Published, JBC Papers in Press, July 23, 2014, DOI 10.1074/jbc.M114.592592 David Frescas and Titia de Lange1 From the Laboratory for Cell Biology and Genetics, The Rockefeller University, New York, New York 10065 Background: Chromosome ends require the TPP1/POT1 heterodimers for protection. Results: A TIN2 mutant that fails to bind TPP1 resulted in phenotypes associated with TPP1/POT1 deletion. -

Catalog and Student & Faculty Handbook
CATALOG AND STUDENT & FACULTY HANDBOOK Leadership & Staff Kenneth J. Marians, Ph.D., Dean Linda D. Burnley, Associate Dean David L. McDonagh, Registrar/Curriculum Specialist Yuliya Masen, Admissions Coordinator Alexandria R. Woodside, Administrative Assistant The Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer Center, is accredited by the New York State Board of Regents and the Commissioner of Education, located at 89 Washington Avenue, Albany, New York 12234; Telephone: (518) 474-1551. 2 Table of Contents Introduction ................................................................................................................................... 4 The Cancer Biology Ph.D. Program ......................................................................................... 4 Admissions ...................................................................................................................................... 6 Transfer of credit .......................................................................................................................... 6 Financial Support .......................................................................................................................... 7 Stipend and Tuition ...................................................................................................................... 7 Supplemental Stipend Support .................................................................................................... 7 First Year Allowance -

2020 CCSS Progress Report
PARTICIPATING CENTERS St. Jude Children's Research Hospital Progress Report Memphis, TN Ann & Robert Lurie Children’s Hospital of Chicago 2020 Chicago, IL Children's Healthcare of Atlanta/Emory University Atlanta, GA Children's Hospital at Stanford Palo Alto, CA Children's Hospital Colorado Aurora, CO Children's Hospital of Los Angeles Los Angeles, CA Children’s Hospital of Orange County Orange, CA Children's Hospital of Philadelphia Philadelphia, PA Children's Hospital of Pittsburgh Pittsburgh, PA Children's Hospitals and Clinics of Minnesota Minneapolis, MN Children's National Medical Center Washington, DC City of Hope National Medical Center Duarte, CA Cook Children’s Hematology-Oncology Center Fort Worth, TX Dana-Farber Cancer Institute/Children’s Hospital Boston, MA Mattel Children’s Hospital at UCLA Los Angeles, CA Mayo Clinic Rochester, MN Memorial Sloan-Kettering Cancer Center NCI-funded resource for outcome New York, NY and intervention research Miller Children's Hospital Long Beach, CA Nationwide Children’s Hospital Columbus, OH U24 CA55727 Riley Hospital for Children - Indiana University Indianapolis, IN Roswell Park Cancer Institute Buffalo, NY Seattle Children's Hospital Seattle, WA St. Louis Children's Hospital St. Louis, MO Texas Children's Hospital Houston, TX Toronto Hospital for Sick Children Toronto, ON UAB/The Children's Hospital of Alabama Birmingham, AL University of California at San Francisco San Francisco, CA University of Chicago Comer Children’s Hospital Chicago, IL University of Michigan - Mott Children's Hospital Ann Arbor, MI University of Minnesota Minneapolis, MN UT-Southwestern Medical Center Dallas, TX http://ccss.stjude.org U.T.M.D. -

Student & Faculty Handbook
STUDENT & FACULTY HANDBOOK Staff Kenneth J. Marians, Ph.D., Dean Linda D. Burnley, Associate Dean Maria J. Torres, Office Manager Iwona Abramek, Registrar/Curriculum Specialist Ivan R. Gerena, Sr. Administrative Assistant Alexandria R. Woodside, Administrative Assistant The Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer Center, is accredited by the New York State Board of Regents and the Commissioner of Education, located at 89 Washington Avenue, Albany, New York 12234; Telephone: (518) 474-1551. 2 Table of Contents Introduction ................................................................................................................................... 4 Ph.D. Program .......................................................................................................................... 4 Admissions ..................................................................................................................................... 6 Transfer of credit .......................................................................................................................... 6 Financial Support .......................................................................................................................... 7 Stipend and Tuition ...................................................................................................................... 7 Supplemental Stipend Support ................................................................................................... -

Dual Recognition of H3k4me3 and H3k27me3 by a Plant Histone Reader SHL
ARTICLE DOI: 10.1038/s41467-018-04836-y OPEN Dual recognition of H3K4me3 and H3K27me3 by a plant histone reader SHL Shuiming Qian1,2, Xinchen Lv3,4, Ray N. Scheid1,2,LiLu1,2, Zhenlin Yang3,4, Wei Chen3, Rui Liu3, Melissa D. Boersma2, John M. Denu2,5,6, Xuehua Zhong 1,2 & Jiamu Du 3 The ability of a cell to dynamically switch its chromatin between different functional states constitutes a key mechanism regulating gene expression. Histone mark “readers” display 1234567890():,; distinct binding specificity to different histone modifications and play critical roles in reg- ulating chromatin states. Here, we show a plant-specific histone reader SHORT LIFE (SHL) capable of recognizing both H3K27me3 and H3K4me3 via its bromo-adjacent homology (BAH) and plant homeodomain (PHD) domains, respectively. Detailed biochemical and structural studies suggest a binding mechanism that is mutually exclusive for either H3K4me3 or H3K27me3. Furthermore, we show a genome-wide co-localization of SHL with H3K27me3 and H3K4me3, and that BAH-H3K27me3 and PHD-H3K4me3 interactions are important for SHL-mediated floral repression. Together, our study establishes BAH-PHD cassette as a dual histone methyl-lysine binding module that is distinct from others in recognizing both active and repressive histone marks. 1 Laboratory of Genetics, University of Wisconsin-Madison, Madison, WI 53706, USA. 2 Wisconsin Institute for Discovery, University of Wisconsin-Madison, Madison, WI 53706, USA. 3 National Key Laboratory of Plant Molecular Genetics, CAS Center for Excellence in Molecular Plant Sciences, Shanghai Center for Plant Stress Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 201602, China. -

The Signatures of Autozygosity Among Patients with Colorectal Cancer
Research Article The Signatures of Autozygosity among Patients with Colorectal Cancer Manny D. Bacolod,1 Gunter S. Schemmann,7 Shuang Wang,2 Richard Shattock,1 Sarah F. Giardina,1 Zhaoshi Zeng,3 Jinru Shia,4 Robert F. Stengel,7 Norman Gerry,10 Josephine Hoh,11 Tomas Kirchhoff,5 Bert Gold,9 Michael F. Christman,10 Kenneth Offit,5 William L. Gerald,4 Daniel A. Notterman,8 Jurg Ott,6 Philip B. Paty,3 and Francis Barany1 1Department of Microbiology, Weill Medical College of Cornell University; 2Department of Biostatistics, Mailman School of Public Health, Columbia University; Departments of 3Surgery, 4Pathology, and 5Medicine, Memorial Sloan-Kettering Cancer Center; 6Laboratory of Statistical Genetics, Rockefeller University, New York, New York; 7School of Engineering and Applied Science and 8Department of Molecular Biology, Princeton University, Princeton, New Jersey; 9Laboratory of Genomic Diversity, National Cancer Institute at Frederick, Frederick, Maryland; 10Department of Genetics and Genomics, Boston University, Boston, Massachusetts; and 11School of Public Health, Yale University, New Haven, Connecticut Abstract million people suffered from the disease in 2002, with the majority Previous studies have shown that among populations with a of cases in industrialized countries (2). Genetics aside, the high rate of consanguinity, there is a significant increase in the incidence of CRC correlates with diets rich in fat and calories, prevalence of cancer. Single nucleotide polymorphism (SNP) and low in vegetables, fruits, and fibers as well as alcohol array data (Affymetrix, 50K XbaI) analysis revealed long consumption and smoking (3). Traditionally, CRC cases are divided regions of homozygosity in genomic DNAs taken from tumor into two categories: sporadic and familial (or hereditary; ref. -
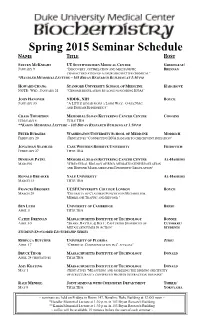
Spring 2015 BCH Sem Sched
Spring 2015 Seminar Schedule Name Title Host STEVEN MCKNIGHT UT SOUTHWESTERN MEDICAL CENTER GREENLEAF/ JANUARY 9 “DISCOVERY, OPTIMIZATION AND MECHANISTIC BRENNAN CHARACTERIZATION OF A NEUROPROTECTIVE CHEMICAL” *HANDLER MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM HOWARD CHANG STANFORD UNIVERSITY SCHOOL OF MEDICINE HARGROVE NOTE: WED., JANUARY 21 “GENOME REGULATION BY LONG NONCODING RNAS” JOHN HANOVER NIDDK, NIH BOYCE JANUARY 30 “A LITTLE SUGAR GOES A LONG WAY: O-GLCNAC AND DISEASE EPIGENETICS” CRAIG THOMPSON MEMORIAL SLOAN KETTERING CANCER CENTER COGGINS FEBRUARY 6 TITLE TBA **KAMIN MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM PETER BURGERS WASHINGTON UNIVERSITY SCHOOL OF MEDICINE MODRICH FEBRUARY 20 (TENTATIVE) “CONNECTING DNA DAMAGE TO CHECKPOINT INITIATION” JONATHAN STAMLER CASE WESTERN RESERVE UNIVERSITY FRIDOVICH FEBRUARY 27 TITLE TBA DINSHAW PATEL MEMORIAL SLOAN KETTERING CANCER CENTER AL-HASHIMI MARCH 6 "STRUCTURAL BIOLOGY OF RNA-MEDIATED GENE REGULATION AND HISTONE MARK-MEDIATED EPIGENETIC REGULATION" RONALD BREAKER YALE UNIVERSITY AL-HASHIMI MARCH 13 TITLE TBA FRANCES BRODSKY UCSF/UNIVERSITY COLLEGE LONDON BOYCE MARCH 20 "DIVERSITY OF CLATHRIN FUNCTION IN METABOLISM, MEMBRANE TRAFFIC AND BEYOND " BEN LUISI UNIVERSITY OF CAMBRIDGE BEESE APRIL 3 TITLE TBA CATHY DRENNAN MASSACHUSETTS INSTITUTE OF TECHNOLOGY BONNIE APRIL 10 "SHAKE, RATTLE, & ROLL: CAPTURING SNAPSHOTS OF CUTHBERT/ METALLOENZYMES IN ACTION" STUDENTS STUDENT-SPONSORED LECTURESHIP SERIES REBECCA BUTCHER UNIVERSITY OF FLORIDA ZHOU APRIL 17 “CHEMICAL COMMUNICATION IN C. ELEGANS” BRUCE TIDOR MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD APRIL 24 (TENTATIVE) TITLE TBA AMY KEATING MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD MAY 1 (TENTATIVE) "MEASURING AND MODELING THE BINDING SPECIFICITY OF STRUCTURALLY CONSERVED PROTEIN INTERACTION DOMAINS" RALF MENDEL JOINT SEMINAR WITH CHEMISTRY DEPARTMENT THIELE/ MAY 8 TITLE TBA YOKOYAMA ~ seminars are held on Fridays in Room 147, Nanaline Duke Building at 12:OO noon ~ *Handler Memorial Lecture at 1:30 p.m. -

Describing the Elephant the Three-Dimensional Structures of Rnas Are Notoriously Difficult to Determine
SCOTT BASKERVILLE AND ANDREW D. ELLINGTON RNA STRUCTURE Describing the elephant The three-dimensional structures of RNAs are notoriously difficult to determine. Functional comparisons of variant molecules and cross-linking experiments are providing new information for structural modeling. The old fable of the blind men trying to describe the in vitro selection is the Rev-binding element (RBE) of shape of an elephant seems aptly to describe the present HIV-1. RBE is a 30-base sequence of the HIV-1 genome state of RNA structural analysis. Until recently, the crys- that can fold into a 'stem-internal-loop-stem' secondary tal structure of only one large RNA, tRNA, was known, structure and that interacts with the Rev protein, and and other non-crystallographic approaches to RNA regulates the splicing and transport of viral mRNAs. structure determination had not been particularly fruit- From a population of partially randomized sequences, ful. Low-resolution electron micrographs are at best able Bartel and Szostak [3] selected RBE variants that could to identify the global structure of the ribosome, but can- bind the Rev protein, whereas Giver et al. [4] selected not provide three-dimensional structural details. The binding variants from a population of completely random nuclear magnetic resonance (NMR) spectra of most sequences. These experiments revealed numerous se- RNAs are so complex that this method of structure quence co-variations that were used by Leclerc et al. [5] determination is limited to molecules of molecular mass 10 000 daltons or less. But new methods now provide at least partial insights into RNA structures. -

Discovery of Common and Rare Genetic Risk Variants for Colorectal Cancer
This is a repository copy of Discovery of common and rare genetic risk variants for colorectal cancer. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/142531/ Version: Accepted Version Article: Huyghe, JR, Bien, SA, Harrison, TA et al. (196 more authors) (2019) Discovery of common and rare genetic risk variants for colorectal cancer. Nature Genetics, 51 (1). pp. 76-87. ISSN 1061-4036 https://doi.org/10.1038/s41588-018-0286-6 © This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply 2018. This is an author produced version of a paper published in Nature Genetics. Uploaded in accordance with the publisher's self-archiving policy. https://doi.org/10.1038/s41588-018-0286-6. Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ 1 Discovery of common and rare genetic risk variants for colorectal