Dual Recognition of H3k4me3 and H3k27me3 by a Plant Histone Reader SHL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Methylation Regulates Discrimination of Enhancers From
Sharifi-Zarchi et al. BMC Genomics (2017) 18:964 DOI 10.1186/s12864-017-4353-7 RESEARCHARTICLE Open Access DNA methylation regulates discrimination of enhancers from promoters through a H3K4me1-H3K4me3 seesaw mechanism Ali Sharifi-Zarchi1,2,3,4†, Daniela Gerovska5†, Kenjiro Adachi6, Mehdi Totonchi3, Hamid Pezeshk7,8, Ryan J. Taft9, Hans R. Schöler6,10, Hamidreza Chitsaz2, Mehdi Sadeghi8,11, Hossein Baharvand3,12* and Marcos J. Araúzo-Bravo5,13,14* Abstract Background: DNA methylation at promoters is largely correlated with inhibition of gene expression. However, the role of DNA methylation at enhancers is not fully understood, although a crosstalk with chromatin marks is expected. Actually, there exist contradictory reports about positive and negative correlations between DNA methylation and H3K4me1, a chromatin hallmark of enhancers. Results: We investigated the relationship between DNA methylation and active chromatin marks through genome- wide correlations, and found anti-correlation between H3K4me1 and H3K4me3 enrichment at low and intermediate DNA methylation loci. We hypothesized “seesaw” dynamics between H3K4me1 and H3K4me3 in the low and intermediate DNA methylation range, in which DNA methylation discriminates between enhancers and promoters, marked by H3K4me1 and H3K4me3, respectively. Low methylated regions are H3K4me3 enriched, while those with intermediate DNA methylation levels are progressively H3K4me1 enriched. Additionally, the enrichment of H3K27ac, distinguishing active from primed enhancers, follows a plateau in the lower range of the intermediate DNA methylation level, corresponding to active enhancers, and decreases linearly in the higher range of the intermediate DNA methylation. Thus, the decrease of the DNA methylation switches smoothly the state of the enhancers from a primed to an active state. -

Modes of Interaction of KMT2 Histone H3 Lysine 4 Methyltransferase/COMPASS Complexes with Chromatin
cells Review Modes of Interaction of KMT2 Histone H3 Lysine 4 Methyltransferase/COMPASS Complexes with Chromatin Agnieszka Bochy ´nska,Juliane Lüscher-Firzlaff and Bernhard Lüscher * ID Institute of Biochemistry and Molecular Biology, Medical School, RWTH Aachen University, Pauwelsstrasse 30, 52057 Aachen, Germany; [email protected] (A.B.); jluescher-fi[email protected] (J.L.-F.) * Correspondence: [email protected]; Tel.: +49-241-8088850; Fax: +49-241-8082427 Received: 18 January 2018; Accepted: 27 February 2018; Published: 2 March 2018 Abstract: Regulation of gene expression is achieved by sequence-specific transcriptional regulators, which convey the information that is contained in the sequence of DNA into RNA polymerase activity. This is achieved by the recruitment of transcriptional co-factors. One of the consequences of co-factor recruitment is the control of specific properties of nucleosomes, the basic units of chromatin, and their protein components, the core histones. The main principles are to regulate the position and the characteristics of nucleosomes. The latter includes modulating the composition of core histones and their variants that are integrated into nucleosomes, and the post-translational modification of these histones referred to as histone marks. One of these marks is the methylation of lysine 4 of the core histone H3 (H3K4). While mono-methylation of H3K4 (H3K4me1) is located preferentially at active enhancers, tri-methylation (H3K4me3) is a mark found at open and potentially active promoters. Thus, H3K4 methylation is typically associated with gene transcription. The class 2 lysine methyltransferases (KMTs) are the main enzymes that methylate H3K4. KMT2 enzymes function in complexes that contain a necessary core complex composed of WDR5, RBBP5, ASH2L, and DPY30, the so-called WRAD complex. -

Modeling of Histone Modifications Reveals Formation Mechanism and Function of Bivalent Chromatin
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.03.429504; this version posted February 3, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Modeling of histone modifications reveals formation mechanism and function of bivalent chromatin Wei Zhao1,2, Lingxia Qiao1, Shiyu Yan2, Qing Nie3,*, Lei Zhang1,2,* 1 Beijing International Center for Mathematical Research, Peking University, Beijing 100871, China 2 Center for Quantitative Biology, Peking University, Beijing 100871, China 3 Department of Mathematics and Department of Developmental and Cell Biology, University of California Irvine, Irvine, CA 92697, USA. *Corresponding authors: Qing Nie, email: [email protected]; Lei Zhang, email: [email protected] Abstract Bivalent chromatin is characterized by occupation of both activating histone modifications and repressive histone modifications. While bivalent chromatin is known to link with many biological processes, the mechanisms responsible for its multiple functions remain unclear. Here, we develop a mathematical model that involves antagonistic histone modifications H3K4me3 and H3K27me3 to capture the key features of bivalent chromatin. Three necessary conditions for the emergence of bivalent chromatin are identified, including advantageous methylating activity over demethylating activity, frequent noise conversions of modifications, and sufficient nonlinearity. The first condition is further confirmed by analyzing the -

Epigenetic Regulation of Promiscuous Gene Expression in Thymic Medullary Epithelial Cells
Epigenetic regulation of promiscuous gene expression in thymic medullary epithelial cells Lars-Oliver Tykocinskia,1,2, Anna Sinemusa,1, Esmail Rezavandya, Yanina Weilandb, David Baddeleyb, Christoph Cremerb, Stephan Sonntagc, Klaus Willeckec, Jens Derbinskia, and Bruno Kyewskia,3 aDivision of Developmental Immunology, Tumor Immunology Program, German Cancer Research Center, D-69120 Heidelberg, Germany; bKirchhoff Institute for Physics, University of Heidelberg, D-69120 Heidelberg, Germany; and cInstitute for Genetics, University of Bonn, D-53117 Bonn, Germany Edited* by Philippa Marrack, National Jewish Health, Denver, CO, and approved September 28, 2010 (received for review July 2, 2010) Thymic central tolerance comprehensively imprints the T-cell re- ing of delimited regions allowing access of general and specific ceptor repertoire before T cells seed the periphery. Medullary transcriptional factors to act on gene-specific control elements thymic epithelial cells (mTECs) play a pivotal role in this process by (8). This scenario is clearly different from the intricate regulation virtue of promiscuous expression of tissue-restricted autoantigens. of functionally related gene families like the Hox gene locus or β The molecular regulation of this unusual gene expression, in the -globin gene locus (9). A similar phenomenon as observed in Drosophila particular the involvement of epigenetic mechanisms is only poorly has been reported for housekeeping genes but not for understood. By studying promiscuous expression of the mouse TRAs in vertebrates (10). casein locus, we report that transcription of this locus proceeds Here we analyzed the interrelationship between emerging gene expression patterns at the single cell level, promoter-associated from a delimited region (“entry site”) to increasingly complex pat- epigenetic marks, and the differentiation of mTECs in the murine terns along with mTEC maturation. -

2012 Annual Report
Memorial Sloan-Kettering Cancer Center 2012 ANNUAL REPORT A SHARED VISION A SINGULAR MISSION Nurse practitioner Naomi Cazeau, of the Adult Bone Marrow Transplant Service. PING CHI PHYSICIAN-SCIENTIST 10 STEPHEN SOLOMON ALEXANDER RUDENSKY INTERVENTIONAL IMMUNOLOGIST RADIOLOGIST 16 12 VIVIANE TABAR The clinicians and scientists of NEUROSURGEON Memorial Sloan-Kettering share a vision and 18 a singular mission — to conquer cancer. STEPHEN LONG STRUCTURAL BIOLOGIST They are experts united against a 20 SIMON POWELL complex disease. Each type of cancer R ADIATION ONCOLOGIST 24 ETHEL LAW is different, each tumor is unique. Set free NURSE PRACTITIONER in surroundings that invite the sharing of 26 ideas and resources, they attack the CHRISTINA LESLIE complexity of cancer from every angle COMPUTATIONAL BIOLOGIST and every discipline. 34 SCOTT ARMSTRONG PEDIATRIC ONCOLOGIST 30 TO JORGE REIS-FILHO EXPERIMENTAL PATHOLOGIST CONQUER 38 CANCER 04 Letter from the Chairman and the President A complete version of this report — 42 Statistical Profile which includes lists of our donors, 44 Financial Summary doctors, and scientists — 46 Boards of Overseers and Managers is available on our website at 49 The Campaign for Memorial Sloan-Kettering www.mskcc.org/annualreport. 4 5 Letter from the Chairman In 2012 the leadership of Memorial Sloan-Kettering endorsed Douglas A. Warner III These programmatic investments require leadership and and the President a $2.2 billion investment in a clinical expansion that will set vision. Our new Physician-in-Chief, José Baselga, joined the stage for a changing care paradigm into the next decade us on January 1, 2013. An internationally recognized and beyond. -

2011 Annual Report
Memorial Sloan-Kettering Cancer Center 2011 Annual Report 10 steps closer 10 steps closer Letter from the Chairman and the President 1 1 | First effective treatments for advanced melanoma 5 2 Genomic analysis offers clues to most common | type of ovarian cancer 7 3 Breast cancer surgery: practice-changing | findings for some patients 9 4 New drugs offer survival benefit for men | with metastatic prostate cancer 11 5 | Insights into DNA damage and repair 13 6 Novel stem cell technique shows promise | in treating disease 15 7 Combination therapy may prevent spread | of nasopharyngeal tumors 17 8 Algorithm can predict shape of proteins, | speeding basic cancer research 19 9 Two of 2011’s top five advances in cancer | research led by MSKCC physician-scientists 21 10 | The Josie Robertson Surgery Center 23 The Campaign for Memorial Sloan-Kettering 25 Statistical Profile 27 Financial Summary 29 Boards of Overseers and Managers 31 www.mskcc.org/annualreport Letter from the Chairman and the President The year 2011 was a strong one at Memorial Sloan-Kettering. We continued to lead across “Our success as an institution is due in the spectrum of patient care, research, and training, and laid the groundwork for important progress in the years ahead. great measure to our remarkable staff… We want to begin by saying that our success as an institution is due in great measure to our remarkable staff. On a daily basis, we are inspired by their dedication and compassion, and We are inspired by their dedication Douglas A. Warner III are grateful for the work they do in the service of our patients and our mission. -

H3k4me3 Is Neither Instructive For, Nor Informed By, Transcription
bioRxiv preprint first posted online Jul. 19, 2019; doi: http://dx.doi.org/10.1101/709014. The copyright holder for this preprint (which was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license. H3K4me3 is neither instructive for, nor informed by, transcription. Struan C Murray1, Philipp Lorenz1, Françoise S Howe1, Meredith Wouters1, Thomas Brown1, Shidong Xi1, Harry Fischl1, Walaa Khushaim1, Joseph Regish Rayappu1, Andrew Angel1 & Jane Mellor1* 1Department of Biochemistry, University of Oxford, Oxford, OX1 3QU, UK. *Corresponding author. Tel: +44 1865 613241; E-mail: [email protected] Key words histone modification; gene regulation; H3K4me3; chromatin; RNA polymerase Abstract H3K4me3 is a near-universal histone modification found predominantly at the 5’ region of genes, with a well-documented association with gene activity. H3K4me3 has been ascribed roles as both an instructor of gene expression and also a downstream consequence of expression, yet neither has been convincingly proven on a genome-wide scale. Here we test these relationships using a combination of bioinformatics, modelling and experimental data from budding yeast in which the levels of H3K4me3 have been massively ablated. We find that loss of H3K4me3 has no effect on the levels of nascent transcription or transcript in the population. Moreover, we observe no change in the rates of transcription initiation, elongation, mRNA export or turnover, or in protein levels, or cell-to-cell variation of mRNA. Loss of H3K4me3 also has no effect on the large changes in gene expression patterns that follow galactose induction. -

Histone H3k27me3 Demethylases Regulate Human Th17 Cell Development and Effector Functions by Impacting on Metabolism
Histone H3K27me3 demethylases regulate human Th17 cell development and effector functions by impacting on metabolism Adam P. Cribbsa,1, Stefan Terlecki-Zaniewicza, Martin Philpotta, Jeroen Baardmanb, David Ahernc, Morten Lindowd, Susanna Obadd, Henrik Oerumd, Brante Sampeye, Palwinder K. Manderf, Henry Penng, Paul Wordswortha, Paul Bownessa, Menno de Wintherh, Rab K. Prinjhaf, Marc Feldmanna,c,1, and Udo Oppermanna,i,j,k,1 aBotnar Research Center, Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences, National Institute for Health Research Oxford Biomedical Research Unit, University of Oxford, OX3 7LD Oxford, United Kingdom; bExperimental Vascular Biology, Department of Medical Biochemistry, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centres, University of Amsterdam, 1105AZ Amsterdam, The Netherlands; cKennedy Institute of Rheumatology, Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences, National Institute for Health Research Oxford Biomedical Research Unit, University of Oxford, OX3 7FY Oxford, United Kingdom; dRoche Innovation Center Copenhagen A/S, DK 2970 Hørsholm, Denmark; eMetabolon Inc., Durham, NC 27713; fEpinova Discovery Performance Unit, Medicines Research Centre, GlaxoSmithKline R&D, SG1 2NY Stevenage, United Kingdom; gArthritis Centre, Northwick Park Hospital, HA13UJ Harrow, United Kingdom; hInstitute for Cardiovascular Prevention, Ludwig Maximilians University, 80336 Munich, Germany; iStructural Genomics Consortium, University of Oxford, OX3 7DQ Oxford, -

Direct Interactions Promote Eviction of the Sir3 Heterochromatin Protein by the SWI/SNF Chromatin Remodeling Enzyme
Direct interactions promote eviction of the Sir3 heterochromatin protein by the SWI/SNF chromatin remodeling enzyme Benjamin J. Manning and Craig L. Peterson1 Program in Molecular Medicine, University of Massachusetts Medical School, Worcester, MA 01605 Edited by Jasper Rine, University of California, Berkeley, CA, and approved November 11, 2014 (received for review October 20, 2014) Heterochromatin is a specialized chromatin structure that is central the Rsc2p subunit of the remodels structure of chromatin (RSC) to eukaryotic transcriptional regulation and genome stability. remodeling enzyme and the Orc1p subunit of the Origin Recogni- Despite its globally repressive role, heterochromatin must also tion Complex (ORC) (14). The stability of the Sir3p BAH–nucle- be dynamic, allowing for its repair and replication. In budding osome complex requires deacetylated histone H4 lysine 16 (15); yeast, heterochromatin formation requires silent information consequently, amino acid substitutions at H4-K16 disrupt Sir3p– regulators (Sirs) Sir2p, Sir3p, and Sir4p, and these Sir proteins nucleosome binding and eliminate heterochromatin assembly in create specialized chromatin structures at telomeres and silent vivo (15–17). mating-type loci. Previously, we found that the SWI/SNF chromatin Despite the repressive structure of heterochromatin, these remodeling enzyme can catalyze the ATP-dependent eviction of domains must be replicated and repaired, implying that mecha- Sir3p from recombinant nucleosomal arrays, and this activity nisms exist to regulate heterochromatin disassembly. Previously, enhances early steps of recombinational repair in vitro. Here, we we described an in vitro assay to monitor early steps of re- show that the ATPase subunit of SWI/SNF, Swi2p/Snf2p, interacts combinational repair with recombinant nucleosomal array sub- with the heterochromatin structural protein Sir3p. -

The Importance of Epigenetics in Diagnostics and Treatment of Major Depressive Disorder
Journal of Personalized Medicine Review The Importance of Epigenetics in Diagnostics and Treatment of Major Depressive Disorder Piotr Czarny 1,† , Katarzyna Białek 2,†, Sylwia Ziółkowska 1,†, Justyna Strycharz 1 , Gabriela Barszczewska 2 and Tomasz Sliwinski 2,* 1 Department of Medical Biochemistry, Medical University of Lodz, 92-216 Lodz, Poland; [email protected] (P.C.); [email protected] (S.Z.); [email protected] (J.S.) 2 Laboratory of Medical Genetics, Faculty of Biology and Environmental Protection, University of Lodz, 90-236 Lodz, Poland; [email protected] (K.B.); [email protected] (G.B.) * Correspondence: [email protected] † Equal contribution. Abstract: Recent studies imply that there is a tight association between epigenetics and a molecular mechanism of major depressive disorder (MDD). Epigenetic modifications, i.e., DNA methylation, post-translational histone modification and interference of microRNA (miRNA) or long non-coding RNA (lncRNA), are able to influence the severity of the disease and the outcome of the therapy. This article summarizes the most recent literature data on this topic, i.e., usage of histone deacetylases as therapeutic agents with an antidepressant effect and miRNAs or lncRNAs as markers of depression. Due to the noteworthy potential of the role of epigenetics in MDD diagnostics and therapy, we have gathered the most relevant data in this area. Citation: Czarny, P.; Białek, K.; Ziółkowska, S.; Strycharz, J.; Keywords: depression; epigenetics; personalized medicine; histones; histone modifications; DNA Barszczewska, G.; Sliwinski, T. The methylation; microRNA; long non-coding RNA Importance of Epigenetics in Diagnostics and Treatment of Major Depressive Disorder. -

Intact Nucleosomal Context Enables Chromodomain Reader
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.30.070136; this version posted April 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Intact nucleosomal context enables chromodomain reader MRG15 to distinguish H3K36me3 from -me2 Sarah Faulkner1,2, Antony M. Couturier2, Brian Josephson1, Tom Watts1, Benjamin G. Davis1,3# and Fumiko Esashi2# 1 Department of Chemistry, University of Oxford, 12 Mansfield Rd, Oxford OX1 3TA, United Kingdom 2 Sir William Dunn School of Pathology, University of Oxford, S Parks Rd, Oxford OX1 3RE, United Kingdom 3 The Rosalind Franklin Institute, Oxfordshire, OX11 0FA, United Kingdom #To whom correspondence should be addressed: [email protected] and [email protected] Keywords: chromodomain, H3K36 methylation, MRG15, nucleosome, SETD2 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.30.070136; this version posted April 30, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract A wealth of in vivo evidence demonstrates the physiological importance of histone H3 trimethylation at lysine 36 (H3K36me3), to which chromodomain-containing proteins, such as MRG15, bind preferentially compared to their dimethyl (H3K36me2) counterparts. However, in vitro studies using isolated H3 peptides have failed to recapitulate a causal interaction. Here, we show that MRG15 can clearly discriminate between synthetic, fully intact model nucleosomes containing H3K36me2 and H3K36me3. MRG15 docking studies, along with experimental observations and nucleosome structure analysis suggest a model where the H3K36 side chain is sequestered in intact nucleosomes via a hydrogen bonding interaction with the DNA backbone, which is abrogated when the third methyl group is added to form H3K36me3. -
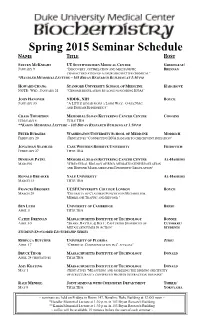
Spring 2015 BCH Sem Sched
Spring 2015 Seminar Schedule Name Title Host STEVEN MCKNIGHT UT SOUTHWESTERN MEDICAL CENTER GREENLEAF/ JANUARY 9 “DISCOVERY, OPTIMIZATION AND MECHANISTIC BRENNAN CHARACTERIZATION OF A NEUROPROTECTIVE CHEMICAL” *HANDLER MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM HOWARD CHANG STANFORD UNIVERSITY SCHOOL OF MEDICINE HARGROVE NOTE: WED., JANUARY 21 “GENOME REGULATION BY LONG NONCODING RNAS” JOHN HANOVER NIDDK, NIH BOYCE JANUARY 30 “A LITTLE SUGAR GOES A LONG WAY: O-GLCNAC AND DISEASE EPIGENETICS” CRAIG THOMPSON MEMORIAL SLOAN KETTERING CANCER CENTER COGGINS FEBRUARY 6 TITLE TBA **KAMIN MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM PETER BURGERS WASHINGTON UNIVERSITY SCHOOL OF MEDICINE MODRICH FEBRUARY 20 (TENTATIVE) “CONNECTING DNA DAMAGE TO CHECKPOINT INITIATION” JONATHAN STAMLER CASE WESTERN RESERVE UNIVERSITY FRIDOVICH FEBRUARY 27 TITLE TBA DINSHAW PATEL MEMORIAL SLOAN KETTERING CANCER CENTER AL-HASHIMI MARCH 6 "STRUCTURAL BIOLOGY OF RNA-MEDIATED GENE REGULATION AND HISTONE MARK-MEDIATED EPIGENETIC REGULATION" RONALD BREAKER YALE UNIVERSITY AL-HASHIMI MARCH 13 TITLE TBA FRANCES BRODSKY UCSF/UNIVERSITY COLLEGE LONDON BOYCE MARCH 20 "DIVERSITY OF CLATHRIN FUNCTION IN METABOLISM, MEMBRANE TRAFFIC AND BEYOND " BEN LUISI UNIVERSITY OF CAMBRIDGE BEESE APRIL 3 TITLE TBA CATHY DRENNAN MASSACHUSETTS INSTITUTE OF TECHNOLOGY BONNIE APRIL 10 "SHAKE, RATTLE, & ROLL: CAPTURING SNAPSHOTS OF CUTHBERT/ METALLOENZYMES IN ACTION" STUDENTS STUDENT-SPONSORED LECTURESHIP SERIES REBECCA BUTCHER UNIVERSITY OF FLORIDA ZHOU APRIL 17 “CHEMICAL COMMUNICATION IN C. ELEGANS” BRUCE TIDOR MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD APRIL 24 (TENTATIVE) TITLE TBA AMY KEATING MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD MAY 1 (TENTATIVE) "MEASURING AND MODELING THE BINDING SPECIFICITY OF STRUCTURALLY CONSERVED PROTEIN INTERACTION DOMAINS" RALF MENDEL JOINT SEMINAR WITH CHEMISTRY DEPARTMENT THIELE/ MAY 8 TITLE TBA YOKOYAMA ~ seminars are held on Fridays in Room 147, Nanaline Duke Building at 12:OO noon ~ *Handler Memorial Lecture at 1:30 p.m.