SFMBT1 Functions with LSD1 to Regulate Expression of Canonical Histone Genes and Chromatin-Related Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome-Wide Analysis of 5-Hmc in the Peripheral Blood of Systemic Lupus Erythematosus Patients Using an Hmedip-Chip
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 35: 1467-1479, 2015 Genome-wide analysis of 5-hmC in the peripheral blood of systemic lupus erythematosus patients using an hMeDIP-chip WEIGUO SUI1*, QIUPEI TAN1*, MING YANG1, QIANG YAN1, HUA LIN1, MINGLIN OU1, WEN XUE1, JIEJING CHEN1, TONGXIANG ZOU1, HUANYUN JING1, LI GUO1, CUIHUI CAO1, YUFENG SUN1, ZHENZHEN CUI1 and YONG DAI2 1Guangxi Key Laboratory of Metabolic Diseases Research, Central Laboratory of Guilin 181st Hospital, Guilin, Guangxi 541002; 2Clinical Medical Research Center, the Second Clinical Medical College of Jinan University (Shenzhen People's Hospital), Shenzhen, Guangdong 518020, P.R. China Received July 9, 2014; Accepted February 27, 2015 DOI: 10.3892/ijmm.2015.2149 Abstract. Systemic lupus erythematosus (SLE) is a chronic, Introduction potentially fatal systemic autoimmune disease characterized by the production of autoantibodies against a wide range Systemic lupus erythematosus (SLE) is a typical systemic auto- of self-antigens. To investigate the role of the 5-hmC DNA immune disease, involving diffuse connective tissues (1) and modification with regard to the onset of SLE, we compared is characterized by immune inflammation. SLE has a complex the levels 5-hmC between SLE patients and normal controls. pathogenesis (2), involving genetic, immunologic and envi- Whole blood was obtained from patients, and genomic DNA ronmental factors. Thus, it may result in damage to multiple was extracted. Using the hMeDIP-chip analysis and valida- tissues and organs, especially the kidneys (3). SLE arises from tion by quantitative RT-PCR (RT-qPCR), we identified the a combination of heritable and environmental influences. differentially hydroxymethylated regions that are associated Epigenetics, the study of changes in gene expression with SLE. -

2012 Annual Report
Memorial Sloan-Kettering Cancer Center 2012 ANNUAL REPORT A SHARED VISION A SINGULAR MISSION Nurse practitioner Naomi Cazeau, of the Adult Bone Marrow Transplant Service. PING CHI PHYSICIAN-SCIENTIST 10 STEPHEN SOLOMON ALEXANDER RUDENSKY INTERVENTIONAL IMMUNOLOGIST RADIOLOGIST 16 12 VIVIANE TABAR The clinicians and scientists of NEUROSURGEON Memorial Sloan-Kettering share a vision and 18 a singular mission — to conquer cancer. STEPHEN LONG STRUCTURAL BIOLOGIST They are experts united against a 20 SIMON POWELL complex disease. Each type of cancer R ADIATION ONCOLOGIST 24 ETHEL LAW is different, each tumor is unique. Set free NURSE PRACTITIONER in surroundings that invite the sharing of 26 ideas and resources, they attack the CHRISTINA LESLIE complexity of cancer from every angle COMPUTATIONAL BIOLOGIST and every discipline. 34 SCOTT ARMSTRONG PEDIATRIC ONCOLOGIST 30 TO JORGE REIS-FILHO EXPERIMENTAL PATHOLOGIST CONQUER 38 CANCER 04 Letter from the Chairman and the President A complete version of this report — 42 Statistical Profile which includes lists of our donors, 44 Financial Summary doctors, and scientists — 46 Boards of Overseers and Managers is available on our website at 49 The Campaign for Memorial Sloan-Kettering www.mskcc.org/annualreport. 4 5 Letter from the Chairman In 2012 the leadership of Memorial Sloan-Kettering endorsed Douglas A. Warner III These programmatic investments require leadership and and the President a $2.2 billion investment in a clinical expansion that will set vision. Our new Physician-in-Chief, José Baselga, joined the stage for a changing care paradigm into the next decade us on January 1, 2013. An internationally recognized and beyond. -

For Reference Only
Datasheet Version: 1.0.0 Revision date: 05 Nov 2020 Histone H3R2me2a Antibody Catalogue No.:abx000029 Western blot analysis of extracts of various cell lines, using Asymmetric Dimethyl-Histone H3- R2 antibody (abx000029). Dot-blot analysis of various methylation peptides using Asymmetric Dimethyl-Histone H3-R2 antibody (abx000029). Immunofluorescence analysis of 293T cells using Asymmetric Dimethyl-Histone H3-R2 antibody (abx000029). Blue: DAPI for nuclear staining. Histone H3R2me2a Antibody is a Rabbit Polyclonal antibody against Histone H3R2me2a. Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Nucleosomes consist of approximately 146 bp of DNA wrapped around a histone octamer composed of pairs of each of the four core histones (H2A, H2B, H3, and H4). The chromatin fiber is further compacted through the interaction of a linker histone, H1, with the DNA between the nucleosomes to form higher order chromatin structures. This gene is intronless and encodes a member of the histone H3 family. TranscriptsFor from this Reference gene lack polyA tails; instead, they contain a palindromic Only termination element. This gene is located separately from the other H3 genes that are in the histone gene cluster on chromosome 6p22-p21.3. Target: Histone H3R2me2a Clonality: Polyclonal Reactivity: Human, Mouse, Rat v1.0.0 Abbexa Ltd, Cambridge, UK · Phone: +44 1223 755950 · Fax: +44 1223 755951 1 Abbexa LLC, Houston, TX, USA · Phone: +1 832 327 7413 www.abbexa.com · Email: [email protected] Datasheet Version: 1.0.0 Revision date: 05 Nov 2020 Tested Applications: WB, IHC, IF/ICC, IP, ChIP Host: Rabbit Recommended dilutions: WB: 1/500 - 1/2000, IHC: 1/50 - 1/200, IF/ICC: 1/50 - 1/200, IP: 1/50 - 1/200, ChIP: 1/20 - 1/100, ChIPseq: 1/20 - 1/100. -

2011 Annual Report
Memorial Sloan-Kettering Cancer Center 2011 Annual Report 10 steps closer 10 steps closer Letter from the Chairman and the President 1 1 | First effective treatments for advanced melanoma 5 2 Genomic analysis offers clues to most common | type of ovarian cancer 7 3 Breast cancer surgery: practice-changing | findings for some patients 9 4 New drugs offer survival benefit for men | with metastatic prostate cancer 11 5 | Insights into DNA damage and repair 13 6 Novel stem cell technique shows promise | in treating disease 15 7 Combination therapy may prevent spread | of nasopharyngeal tumors 17 8 Algorithm can predict shape of proteins, | speeding basic cancer research 19 9 Two of 2011’s top five advances in cancer | research led by MSKCC physician-scientists 21 10 | The Josie Robertson Surgery Center 23 The Campaign for Memorial Sloan-Kettering 25 Statistical Profile 27 Financial Summary 29 Boards of Overseers and Managers 31 www.mskcc.org/annualreport Letter from the Chairman and the President The year 2011 was a strong one at Memorial Sloan-Kettering. We continued to lead across “Our success as an institution is due in the spectrum of patient care, research, and training, and laid the groundwork for important progress in the years ahead. great measure to our remarkable staff… We want to begin by saying that our success as an institution is due in great measure to our remarkable staff. On a daily basis, we are inspired by their dedication and compassion, and We are inspired by their dedication Douglas A. Warner III are grateful for the work they do in the service of our patients and our mission. -

Anti-Histone H3 (Mono Methyl K4) Polyclonal Antibody (CABT-LM00401) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-Histone H3 (mono methyl K4) polyclonal antibody (CABT-LM00401) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Antigen Description Modulation of chromatin structure plays an important role in the regulation of transcription in eukaryotes. The nucleosome, made up of DNA wound around eight core histone proteins (two each of H2A, H2B, H3, and H4), is the primary building block of chroma Target Histone H3K4me2 Immunogen A synthetic methylated peptide corresponding to residues surrounding K4 of human histone H3 Isotype IgG Source/Host Rabbit Species Reactivity Human Purification Antibodies were produced by immunizing rabbits and were purified by antigen affinity- chromatography. Conjugate Unconjugated Applications WB,IHC,IF,IP,ChIP Molecular Weight 15kDa Format Buffer: PBS with 0.02% sodium azide, 50% glycerol, pH7.3. Preservative 0.02% Sodium Azide Storage Store at -20°C or -80°C. Avoid freeze / thaw cycles. GENE INFORMATION Gene Name HIST3H3 histone cluster 3, H3 [ Homo sapiens (human) ] Official Symbol HIST3H3 Synonyms HIST3H3; histone cluster 3, H3; H3 histone family, member T , H3FT, histone 3, H3; histone H3.1t; H3/g; H3t; H3/t; histone 3, H3; H3 histone family, member T; H3.4; H3FT; MGC126886; MGC126888; 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative Diagnostics All Rights Reserved Entrez Gene ID 8290 Protein Refseq NP_003484 UniProt ID Q16695 Chromosome Location 1q42 Pathway Alcoholism; Cell Cycle; Cellular Senescence; Cellular responses to stress; Chromosome Maintenance; DNA Damage/Telomere Stress Induced Senescence; EGFR1 Signaling Pathway; FSH signaling pathway; Meiosis; Meiotic Recombination; Meiotic Synapsis; Packaging O Function DNA binding; protein binding; protein heterodimerization activity References 1. -

The Conserved DNMT1-Dependent Methylation Regions in Human Cells
Freeman et al. Epigenetics & Chromatin (2020) 13:17 https://doi.org/10.1186/s13072-020-00338-8 Epigenetics & Chromatin RESEARCH Open Access The conserved DNMT1-dependent methylation regions in human cells are vulnerable to neurotoxicant rotenone exposure Dana M. Freeman1 , Dan Lou1, Yanqiang Li1, Suzanne N. Martos1 and Zhibin Wang1,2,3* Abstract Background: Allele-specifc DNA methylation (ASM) describes genomic loci that maintain CpG methylation at only one inherited allele rather than having coordinated methylation across both alleles. The most prominent of these regions are germline ASMs (gASMs) that control the expression of imprinted genes in a parent of origin-dependent manner and are associated with disease. However, our recent report reveals numerous ASMs at non-imprinted genes. These non-germline ASMs are dependent on DNA methyltransferase 1 (DNMT1) and strikingly show the feature of random, switchable monoallelic methylation patterns in the mouse genome. The signifcance of these ASMs to human health has not been explored. Due to their shared allelicity with gASMs, herein, we propose that non-tradi- tional ASMs are sensitive to exposures in association with human disease. Results: We frst explore their conservancy in the human genome. Our data show that our putative non-germline ASMs were in conserved regions of the human genome and located adjacent to genes vital for neuronal develop- ment and maturation. We next tested the hypothesized vulnerability of these regions by exposing human embryonic kidney cell HEK293 with the neurotoxicant rotenone for 24 h. Indeed,14 genes adjacent to our identifed regions were diferentially expressed from RNA-sequencing. We analyzed the base-resolution methylation patterns of the predicted non-germline ASMs at two neurological genes, HCN2 and NEFM, with potential to increase the risk of neurodegenera- tion. -

Dual Recognition of H3k4me3 and H3k27me3 by a Plant Histone Reader SHL
ARTICLE DOI: 10.1038/s41467-018-04836-y OPEN Dual recognition of H3K4me3 and H3K27me3 by a plant histone reader SHL Shuiming Qian1,2, Xinchen Lv3,4, Ray N. Scheid1,2,LiLu1,2, Zhenlin Yang3,4, Wei Chen3, Rui Liu3, Melissa D. Boersma2, John M. Denu2,5,6, Xuehua Zhong 1,2 & Jiamu Du 3 The ability of a cell to dynamically switch its chromatin between different functional states constitutes a key mechanism regulating gene expression. Histone mark “readers” display 1234567890():,; distinct binding specificity to different histone modifications and play critical roles in reg- ulating chromatin states. Here, we show a plant-specific histone reader SHORT LIFE (SHL) capable of recognizing both H3K27me3 and H3K4me3 via its bromo-adjacent homology (BAH) and plant homeodomain (PHD) domains, respectively. Detailed biochemical and structural studies suggest a binding mechanism that is mutually exclusive for either H3K4me3 or H3K27me3. Furthermore, we show a genome-wide co-localization of SHL with H3K27me3 and H3K4me3, and that BAH-H3K27me3 and PHD-H3K4me3 interactions are important for SHL-mediated floral repression. Together, our study establishes BAH-PHD cassette as a dual histone methyl-lysine binding module that is distinct from others in recognizing both active and repressive histone marks. 1 Laboratory of Genetics, University of Wisconsin-Madison, Madison, WI 53706, USA. 2 Wisconsin Institute for Discovery, University of Wisconsin-Madison, Madison, WI 53706, USA. 3 National Key Laboratory of Plant Molecular Genetics, CAS Center for Excellence in Molecular Plant Sciences, Shanghai Center for Plant Stress Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 201602, China. -

A Mutation in Histone H2B Represents a New Class of Oncogenic Driver
Author Manuscript Published OnlineFirst on July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. A Mutation in Histone H2B Represents A New Class Of Oncogenic Driver Richard L. Bennett1, Aditya Bele1, Eliza C. Small2, Christine M. Will2, Behnam Nabet3, Jon A. Oyer2, Xiaoxiao Huang1,9, Rajarshi P. Ghosh4, Adrian T. Grzybowski5, Tao Yu6, Qiao Zhang7, Alberto Riva8, Tanmay P. Lele7, George C. Schatz9, Neil L. Kelleher9 Alexander J. Ruthenburg5, Jan Liphardt4 and Jonathan D. Licht1 * 1 Division of Hematology/Oncology, University of Florida Health Cancer Center, Gainesville, FL 2 Division of Hematology/Oncology, Northwestern University 3 Department of Cancer Biology, Dana Farber Cancer Institute and Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School 4 Department of Bioengineering, Stanford University 5 Department of Molecular Genetics and Cell Biology, The University of Chicago 6 Department of Chemistry, Tennessee Technological University 7 Department of Chemical Engineering, University of Florida 8 Bioinformatics Core, Interdisciplinary Center for Biotechnology Research, University of Florida 9 Department of Chemistry, Northwestern University, Evanston IL 60208 Running title: Histone mutations in cancer *Corresponding Author: Jonathan D. Licht, MD The University of Florida Health Cancer Center Cancer and Genetics Research Complex, Suite 145 2033 Mowry Road Gainesville, FL 32610 352-273-8143 [email protected] Disclosures: The authors have no conflicts of interest to declare Downloaded from cancerdiscovery.aacrjournals.org on September 27, 2021. © 2019 American Association for Cancer Research. Author Manuscript Published OnlineFirst on July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -
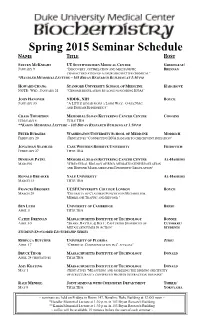
Spring 2015 BCH Sem Sched
Spring 2015 Seminar Schedule Name Title Host STEVEN MCKNIGHT UT SOUTHWESTERN MEDICAL CENTER GREENLEAF/ JANUARY 9 “DISCOVERY, OPTIMIZATION AND MECHANISTIC BRENNAN CHARACTERIZATION OF A NEUROPROTECTIVE CHEMICAL” *HANDLER MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM HOWARD CHANG STANFORD UNIVERSITY SCHOOL OF MEDICINE HARGROVE NOTE: WED., JANUARY 21 “GENOME REGULATION BY LONG NONCODING RNAS” JOHN HANOVER NIDDK, NIH BOYCE JANUARY 30 “A LITTLE SUGAR GOES A LONG WAY: O-GLCNAC AND DISEASE EPIGENETICS” CRAIG THOMPSON MEMORIAL SLOAN KETTERING CANCER CENTER COGGINS FEBRUARY 6 TITLE TBA **KAMIN MEMORIAL LECTURE – 103 BRYAN RESEARCH BUILDING AT 1:30 PM PETER BURGERS WASHINGTON UNIVERSITY SCHOOL OF MEDICINE MODRICH FEBRUARY 20 (TENTATIVE) “CONNECTING DNA DAMAGE TO CHECKPOINT INITIATION” JONATHAN STAMLER CASE WESTERN RESERVE UNIVERSITY FRIDOVICH FEBRUARY 27 TITLE TBA DINSHAW PATEL MEMORIAL SLOAN KETTERING CANCER CENTER AL-HASHIMI MARCH 6 "STRUCTURAL BIOLOGY OF RNA-MEDIATED GENE REGULATION AND HISTONE MARK-MEDIATED EPIGENETIC REGULATION" RONALD BREAKER YALE UNIVERSITY AL-HASHIMI MARCH 13 TITLE TBA FRANCES BRODSKY UCSF/UNIVERSITY COLLEGE LONDON BOYCE MARCH 20 "DIVERSITY OF CLATHRIN FUNCTION IN METABOLISM, MEMBRANE TRAFFIC AND BEYOND " BEN LUISI UNIVERSITY OF CAMBRIDGE BEESE APRIL 3 TITLE TBA CATHY DRENNAN MASSACHUSETTS INSTITUTE OF TECHNOLOGY BONNIE APRIL 10 "SHAKE, RATTLE, & ROLL: CAPTURING SNAPSHOTS OF CUTHBERT/ METALLOENZYMES IN ACTION" STUDENTS STUDENT-SPONSORED LECTURESHIP SERIES REBECCA BUTCHER UNIVERSITY OF FLORIDA ZHOU APRIL 17 “CHEMICAL COMMUNICATION IN C. ELEGANS” BRUCE TIDOR MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD APRIL 24 (TENTATIVE) TITLE TBA AMY KEATING MASSACHUSETTS INSTITUTE OF TECHNOLOGY DONALD MAY 1 (TENTATIVE) "MEASURING AND MODELING THE BINDING SPECIFICITY OF STRUCTURALLY CONSERVED PROTEIN INTERACTION DOMAINS" RALF MENDEL JOINT SEMINAR WITH CHEMISTRY DEPARTMENT THIELE/ MAY 8 TITLE TBA YOKOYAMA ~ seminars are held on Fridays in Room 147, Nanaline Duke Building at 12:OO noon ~ *Handler Memorial Lecture at 1:30 p.m. -

Describing the Elephant the Three-Dimensional Structures of Rnas Are Notoriously Difficult to Determine
SCOTT BASKERVILLE AND ANDREW D. ELLINGTON RNA STRUCTURE Describing the elephant The three-dimensional structures of RNAs are notoriously difficult to determine. Functional comparisons of variant molecules and cross-linking experiments are providing new information for structural modeling. The old fable of the blind men trying to describe the in vitro selection is the Rev-binding element (RBE) of shape of an elephant seems aptly to describe the present HIV-1. RBE is a 30-base sequence of the HIV-1 genome state of RNA structural analysis. Until recently, the crys- that can fold into a 'stem-internal-loop-stem' secondary tal structure of only one large RNA, tRNA, was known, structure and that interacts with the Rev protein, and and other non-crystallographic approaches to RNA regulates the splicing and transport of viral mRNAs. structure determination had not been particularly fruit- From a population of partially randomized sequences, ful. Low-resolution electron micrographs are at best able Bartel and Szostak [3] selected RBE variants that could to identify the global structure of the ribosome, but can- bind the Rev protein, whereas Giver et al. [4] selected not provide three-dimensional structural details. The binding variants from a population of completely random nuclear magnetic resonance (NMR) spectra of most sequences. These experiments revealed numerous se- RNAs are so complex that this method of structure quence co-variations that were used by Leclerc et al. [5] determination is limited to molecules of molecular mass 10 000 daltons or less. But new methods now provide at least partial insights into RNA structures. -

A Mutation in Histone H2B Represents a New Class of Oncogenic Driver
Published OnlineFirst July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 RESEARCH ARTICLE A Mutation in Histone H2B Represents a New Class of Oncogenic Driver Richard L. Bennett1, Aditya Bele1, Eliza C. Small2, Christine M. Will2, Behnam Nabet3, Jon A. Oyer2, Xiaoxiao Huang1,4, Rajarshi P. Ghosh5, Adrian T. Grzybowski6, Tao Yu7, Qiao Zhang8, Alberto Riva9, Tanmay P. Lele8, George C. Schatz4, Neil L. Kelleher4, Alexander J. Ruthenburg6, Jan Liphardt5, and Jonathan D. Licht1 Downloaded from cancerdiscovery.aacrjournals.org on September 30, 2021. © 2019 American Association for Cancer Research. Published OnlineFirst July 23, 2019; DOI: 10.1158/2159-8290.CD-19-0393 ABSTRACT By examination of the cancer genomics database, we identified a new set of mutations in core histones that frequently recur in cancer patient samples and are predicted to disrupt nucleosome stability. In support of this idea, we characterized a glutamate to lysine mutation of histone H2B at amino acid 76 (H2B-E76K), found particularly in bladder and head and neck cancers, that disrupts the interaction between H2B and H4. Although H2B-E76K forms dimers with H2A, it does not form stable histone octamers with H3 and H4 in vitro, and when recon- stituted with DNA forms unstable nucleosomes with increased sensitivity to nuclease. Expression of the equivalent H2B mutant in yeast restricted growth at high temperature and led to defective nucleosome-mediated gene repression. Significantly, H2B-E76K expression in the normal mammary epithelial cell line MCF10A increased cellular proliferation, cooperated with mutant PIK3CA to pro- mote colony formation, and caused a significant drift in gene expression and fundamental changes in chromatin accessibility, particularly at gene regulatory elements. -

TRIM11 Locus Modifies PSP Phenotype
Variation at the TRIM11 locus modifies Progressive Supranuclear Palsy phenotype Article (Accepted Version) Jabbari, Edwin, Woodside, John, Tan, Manueal M X, Shoai, Maryam, Pittman, Alan, Ferrari, Raffaele, Mok, Kin Y, Zhang, David, Reynolds, Regina H, de Silva, Rohan, Grimm, Max-Joseph, Respondek, Gesine, Müller, Ulrich, Al-Sarraj, Safa, Gentleman, Stephen M et al. (2019) Variation at the TRIM11 locus modifies Progressive Supranuclear Palsy phenotype. Annals of Neurology, 84 (4). pp. 485-496. ISSN 0364-5134 This version is available from Sussex Research Online: http://sro.sussex.ac.uk/id/eprint/77646/ This document is made available in accordance with publisher policies and may differ from the published version or from the version of record. If you wish to cite this item you are advised to consult the publisher’s version. Please see the URL above for details on accessing the published version. Copyright and reuse: Sussex Research Online is a digital repository of the research output of the University. Copyright and all moral rights to the version of the paper presented here belong to the individual author(s) and/or other copyright owners. To the extent reasonable and practicable, the material made available in SRO has been checked for eligibility before being made available. Copies of full text items generally can be reproduced, displayed or performed and given to third parties in any format or medium for personal research or study, educational, or not-for-profit purposes without prior permission or charge, provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way.