Thymus-Pineal Gland Axis: Revisiting Its Role in Human Life and Ageing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Circadian Disruption: What Do We Actually Mean?
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Eur J Neurosci Manuscript Author . Author manuscript; Manuscript Author available in PMC 2020 May 07. Circadian disruption: What do we actually mean? Céline Vetter Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA Abstract The circadian system regulates physiology and behavior. Acute challenges to the system, such as those experienced when traveling across time zones, will eventually result in re-synchronization to the local environmental time cues, but this re-synchronization is oftentimes accompanied by adverse short-term consequences. When such challenges are experienced chronically, adaptation may not be achieved, as for example in the case of rotating night shift workers. The transient and chronic disturbance of the circadian system is most frequently referred to as “circadian disruption”, but many other terms have been proposed and used to refer to similar situations. It is now beyond doubt that the circadian system contributes to health and disease, emphasizing the need for clear terminology when describing challenges to the circadian system and their consequences. The goal of this review is to provide an overview of the terms used to describe disruption of the circadian system, discuss proposed quantifications of disruption in experimental and observational settings with a focus on human research, and highlight limitations and challenges of currently available tools. For circadian research to advance as a translational science, clear, operationalizable, and scalable quantifications of circadian disruption are key, as they will enable improved assessment and reproducibility of results, ideally ranging from mechanistic settings, including animal research, to large-scale randomized clinical trials. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

The Interaction of the Thyroid Gland, Pineal Gland and Immune System in Chicken
Vol. 6, Suppl. 2 79 The interaction of the thyroid gland, pineal gland and immune system in chicken Mykola E. Dzerzhynsky1, Olga I. Gorelikova, Andriy S. Pustovalov Department of Cytology, Histology and Development Biology, Kiev, Taras Shevchenko National University, Kiev, Ukraine Received: 7 October 2005; accepted: 15 September 2006 SUMMARY The interaction of immunological system, thyroid and pineal gland was studied in 5-week old males of Gallus domesticus. Several morphometri- cal parameters in pineal and thyroid glands were measured after bird im- munization with human red blood cells and/or treatment with melatonin or seduxen, a melatonin receptor blocker. The peak of the thyroid activity was found on Day 7 after immunization. The immune system appears to directly activate the thyroid gland only in the presence of certain level of melatonin. We suggest that the melatonin mechanism of action includes the enhancement of thyroid gland sensitivity to immune factors. Seduxen prevented the stimulatory influence of the immune system on the thyroid gland. Reproductive Biology 2006, 6, Suppl. 2:79–85. Key words: thyroid gland, immunization, pineal gland, melatonin, seduxen 1Corresponding author: Department of Cytology, Histology and Development Biology, Kiev, Taras Shevchenko National University, 64 Volodomyrska Str, 01033 Kiev, Ukraine; e-mail: [email protected] Copyright © 2006 by the Society for Biology of Reproduction 80 Immune-thyroid-pineal interactions in chicken INTRODUCTION Interrelationships of the endocrine, nervous and immune systems attract a lot of scientific attention [3]. Thyroid hormones (thyroxine: T4; triio- dothyronine), in addition to involvement in controlling energy production and protein and carbohydrate metabolism, stimulate the metamorphosis of lower vertebrates, control tissue growth and development, intensify oxida- tion and heat production as well as influence the functioning of the nervous system. -

Prolongevity Hormone FGF21 Protects Against Immune Senescence by Delaying Age-Related Thymic Involution
Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution Yun-Hee Youma, Tamas L. Horvatha, David J. Mangelsdorfb,c, Steven A. Kliewerb,d, and Vishwa Deep Dixita,e,1 aSection of Comparative Medicine and Program on Integrative Cell Signaling and Neurobiology of Metabolism, Yale School of Medicine, New Haven, CT 06520; bDepartment of Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX 75390; cHoward Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, TX 75390; dDepartment of Molecular Biology, University of Texas Southwestern Medical Center, Dallas, TX 75390; and eDepartment of Immunobiology, Yale School of Medicine, New Haven, CT 06520 Edited by Ruslan Medzhitov, Yale School of Medicine, New Haven, CT, and approved December 16, 2015 (received for review July 22, 2015) Age-related thymic degeneration is associated with loss of naïve T showed that FGF7/keratinocyte growth factor (KGF) adminis- cells, restriction of peripheral T-cell diversity, and reduced health- tration in aged mice partially reversed thymic involution (17–19). span due to lower immune competence. The mechanistic basis of Notably, unlike most FGFs, FGF21 lacks affinity for heparan age-related thymic demise is unclear, but prior evidence suggests sulfate in the extracellular matrix and thus can be secreted to act that caloric restriction (CR) can slow thymic aging by maintaining in an endocrine fashion (20). FGF21 is predominantly secreted thymic epithelial cell integrity and reducing the generation of from liver but is also expressed in thymus (21). FGF21 is a pro- intrathymic lipid. Here we show that the prolongevity ketogenic longevity hormone that elicits it biological effects by binding to β hormone fibroblast growth factor 21 (FGF21), a member of the Klotho in complex with FGF receptor (FGFR) 1c, 2c, or 3c, but endocrine FGF subfamily, is expressed in thymic stromal cells along not FGFR4 (16, 22, 23). -

A Genetically Modified Dermal Micro-Organ Expressing Erythropoietin
(19) & (11) EP 2 377 401 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 19.10.2011 Bulletin 2011/42 A01N 63/00 (2006.01) A01N 65/00 (2009.01) C12N 5/00 (2006.01) C12N 5/02 (2006.01) (2010.01) (21) Application number: 11174205.2 C12N 5/071 (22) Date of filing: 29.04.2004 (84) Designated Contracting States: • Bukhman, Mordechay AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 21891 Carmiel (IL) HU IE IT LI LU MC NL PL PT RO SE SI SK TR • Stern, Baruch, S. Designated Extension States: 34366 Haifa (IL) AL HR LT LV MK • Shalhevet, David 36090 Kiryat Tivon (IL) (30) Priority: 01.05.2003 US 466793 P • Shavitt, Menachem, D. 06.08.2003 US 492754 P 20142 D.N. Misgav (IL) • Pearlman, Andrew, L. (62) Document number(s) of the earlier application(s) in 20164 D.N. Miscav (IL) accordance with Art. 76 EPC: • Noam, Shani 04760621.5 / 1 653 807 30900 Zichron Yaakov (IL) • Almon, Einat (71) Applicant: Medgenics, Inc. 23840 Timrat (IL) Palo Alto, CA 94303 (US) (74) Representative: Modiano, Micaela Nadia (72) Inventors: Modiano & Partners • Bellomo, Stephen, F. Thierschstrasse 11 30900 Zichron Yaakov (IL) 80538 München (DE) • Lippin, Itzhak 42920 Moshav Beit Yitzhak (IL) Remarks: • Piva, Guillermo, Alberto This application was filed on 15-07-2011 as a Winston Salem, NC North Carolina 27104 (US) divisional application to the application mentioned • Rosenberg, Lior under INID code 62. 84965 Omer (IL) (54) A genetically modified dermal micro-organ expressing erythropoietin (57) The present invention is directed to a genetically cro-organ -

And Low-Dose Melatonin Therapies
diseases Review Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies Rüdiger Hardeland Johann Friedrich Blumenbach Institute of Zoology and Anthropology, University of Göttingen, 37073 Göttingen, Germany; [email protected] Abstract: Melatonin has been used preclinically and clinically for different purposes. Some applica- tions are related to readjustment of circadian oscillators, others use doses that exceed the saturation of melatonin receptors MT1 and MT2 and are unsuitable for chronobiological purposes. Conditions are outlined for appropriately applying melatonin as a chronobiotic or for protective actions at elevated levels. Circadian readjustments require doses in the lower mg range, according to receptor affinities. However, this needs consideration of the phase response curve, which contains a silent zone, a delay part, a transition point and an advance part. Notably, the dim light melatonin onset (DLMO) is found in the silent zone. In this specific phase, melatonin can induce sleep onset, but does not shift the circadian master clock. Although sleep onset is also under circadian control, sleep and circadian susceptibility are dissociated at this point. Other limits of soporific effects concern dose, duration of action and poor individual responses. The use of high melatonin doses, up to several hundred mg, for purposes of antioxidative and anti-inflammatory protection, especially in sepsis and viral diseases, have to be seen in the context of melatonin’s tissue levels, its formation in mitochondria, and detoxification of free radicals. Citation: Hardeland, R. Divergent Keywords: circadian; entrainment; inflammation; melatonin; mitochondria; receptor saturation Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies. Diseases 2021, 9, 18. -

Central Immune Senescence, Reversal Potentials
31 Central Immune Senescence, Reversal Potentials Krisztian Kvell and Judit E. Pongracz Department of Medical Biotechnology, University of Pecs, Hungary 1. Introduction 1.1 Ageing in focus Ageing is a complex process that affects all living organisms. Senescence is not only conceivable in multicellular organisms, but also in unicellulars. Unlike certain diseases that have specific morbidity rates, ageing is a physiological process that affects all individuals that live long enough (unaffected by i.e. predation or famine) to experience senescence. A pioneer of ageing research, August Weismann has established two rather opposing concepts for aging and even today both gather numerous followers. One is the adaptive concept, according to which ageing has evolved to cleanse the population from old, non- reproductive consumers. The other, non-adaptive concept suggests that ageing is due to greater weight on early survival / reproduction rather than vigour at later ages. This latter has been reshaped by the theory of antagonistic pleiotropy (Ljubuncic et al. 2009). Due to advances in biomedical research and care, currently an average 55-aged person is expected to live up to 85 years of age at death on average in the Western societies. This number is expected to increase if biomedical research continues to develop at the current rate and by the year 2030 an average 55-aged person is expected to live up to 115 years of age at death (according to SENS plans) (de Grey 2007). If such forecasts prove to be true, it is of extraordinary significance and will likely trigger immense social and economical conflicts. 1.1.1 Ageing and society Ageing of the population is one of the most important challenges for the developed world to face over the next decades. -

The Potential Therapeutic Effect of Melatonin in Gastro-Esophageal Reflux Disease Tharwat S Kandil1*, Amany a Mousa2, Ahmed a El-Gendy3, Amr M Abbas3
Kandil et al. BMC Gastroenterology 2010, 10:7 http://www.biomedcentral.com/1471-230X/10/7 RESEARCH ARTICLE Open Access The potential therapeutic effect of melatonin in gastro-esophageal reflux disease Tharwat S Kandil1*, Amany A Mousa2, Ahmed A El-Gendy3, Amr M Abbas3 Abstract Background: Gastro-Esophageal Reflux Disease (GERD) defined as a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications. Many drugs are used for the treatment of GERD such as omeprazole (a proton pump inhibitor) which is a widely used antiulcer drug demonstrated to protect against esophageal mucosal injury. Melatonin has been found to protect the gastrointestinal mucosa from oxidative damage caused by reactive oxygen species in different experimental ulcer models. The aim of this study is to evaluate the role of exogenous melatonin in the treatment of reflux disease in humans either alone or in combination with omeprazole therapy. Methods: 36 persons were divided into 4 groups (control subjects, patients with reflux disease treated with melatonin alone, omeprazole alone and a combination of melatonin and omeprazole for 4 and 8 weeks) Each group consisted of 9 persons. Persons were subjected to thorough history taking, clinical examination, and investigations including laboratory, endoscopic, record of esophageal motility, pH-metry, basal acid output and serum gastrin. Results: Melatonin has a role in the improvement of Gastro-esophageal reflux disease when used alone or in combination with omeprazole. Meanwhile, omeprazole alone is better used in the treatment of GERD than melatonin alone. Conclusion: The present study showed that oral melatonin is a promising therapeutic agent for the treatment of GERD. -

07. Endocrine, Reproductive and Urogenital Pharmacology 07.001
07. Endocrine, Reproductive and Urogenital Pharmacology 07.001 Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3-Adrenoceptor activation and α1-adrenoceptor blockade. Alexandre EC1, Kiguti LR2, Calmasini FB1, Ferreira R3, Silva FH1, Silva KP2, Ribeiro CA2, Mónica FZ1, Pupo AS2, Antunes E1 1FCM-Unicamp – Farmacologia, 2IBB-Unesp, 3FCM- Unicamp – Hematologia e Hemoterapia Introduction: Overactive bladder syndrome (OAB) is a subset of storage LUTS (lower urinary tract symptoms) highly prevalent in diabetes, obesity and hypertension. Benign prostatic hyperplasia (BPH) in aging men is another pathological condition highly associated with OAB secondary to bladder outlet obstruction (BOO). The β3- adrenoceptor apparently is the major receptor to induce bladder relaxations. Mirabegron is the first β3-adrenoceptor (β3-AR) agonist approved for OAB treatment (Chapple et al., 2014). Urethral smooth muscle plays a critical role to urinary continence, but no studies have examined the mirabegron-induced urethral relaxations. Aims: This study was designed to investigate the mirabegron-induced mouse urethral relaxations. In preliminary assays, mirabegron showed an unexpected action by competitively antagonizing the urethral contractions induced by the α1-AR agonist phenylephrine. Therefore, this study also aimed to characterize the α1-AR blockade by mirabegron, focusing on the α1-AR subtypes in rat vas deferens and prostate (α1A- AR), spleen (α1B-AR) and aorta (α1D-AR) preparations. Methods: Functional assays were carried out in mouse urethra rings, and rat vas deferens, prostate, aorta and spleen. β3-AR expression (mRNA and immunohistochemistry) and cyclic AMP levels were determined in mouse urethra. Competition assays for the specific binding of [3H]Prazosin to membrane preparations of HEK 293 cells expressing each of the human α1-ARs subtypes were performed. -

1-Anatomy of the Pituitary Gland
Color Code Important Anatomy of Pituitary Gland Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of the lecture, students should be able to: ✓ Describe the position of the pituitary gland. ✓ List the structures related to the pituitary gland. ✓ Differentiate between the lobes of the gland. ✓ Describe the blood supply of pituitary gland & the hypophyseal portal system. الغدة النخامية Pituitary Gland (also called Hypophysis Cerebri) o It is referred to as the master of endocrine glands. o It is a small oval structure 1 cm in diameter. o It doubles its size during pregnancy. lactation ,(الحمل) pregnancy ,(الحيض) A women experiences changes in her hormone levels during menstruation But only the pituitary gland will only increase in size during pregnancy .(سن اليأس) and menopause ,(الرضاعة) X-RAY SKULL: LATERAL VIEW SAGITTAL SECTION OF HEAD & NECK Extra Pituitary Gland Position o It lies in the middle cranial fossa. o It is well protected in sella turcica* (hypophyseal fossa) of body of sphenoid o It lies between optic chiasma (anteriorly) & mamillary bodies** (posteriorly). Clinical point: *سرج الحصان Anterior to the pituitary gland is the optic chiasm, so if there was a tumor in the pituitary gland or it was ** Part of hypothalamus enlarged this could press on the chiasm and disrupt the patients vision (loss of temporal field). Extra Pictures The purple part is the sphenoid bone Hypophyseal fossa Pituitary Gland The relations are important Important Relations • SUPERIOR: Diaphragma sellae: A fold of dura mater covers the pituitary gland & has an opening for passage of infundibulum (pituitary stalk) connecting the gland to hypothalamus. -
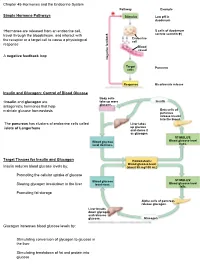
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Glossary of Pediatric Endocrine Terms
Glossary of Pediatric Endocrine Terms Adrenal Glands: Triangle-shaped glands located on top of each kidney that are responsible for the regulation of stress response by producing hormones such as cortisol and adrenaline. Adrenal Crisis: A life-threatening condition that results when there is not enough cortisol (stress hormone) in the body. This may present with nausea, vomiting, abdominal pain, severely low blood pressure, and loss of consciousness. Adrenocorticotropin (ACTH): A hormone produced by the anterior pituitary gland that triggers the adrenal gland to produce cortisol, the stress hormone. Anterior Pituitary: The front of the pituitary gland that secretes growth hormone, gonadotropins, thyroid stimulating hormone, prolactin, adrenocorticotropin hormone. Antidiuretic Hormone: A hormone secreted by the posterior pituitary that controls how much water is excreted by the kidneys (water balance). Bone Age Study: An X-ray of the left hand and wrist to assess a child’s skeletal age. It is often used to evaluate disorders of puberty and growth. Congenital Adrenal Hyperplasia: A group of disorders which impair the adrenal steroid synthesis pathway. This causes the body makes too many androgens. Sometimes the body does not make enough cortisol and aldosterone. Constitutional Delay of Growth and Puberty: A normal variant of growth in which children grow at a slower rate and begin puberty later than their peers. They may appear short until they have catch up growth. They usually end up at a normal adult height. A diagnosis of constitutional delay of growth and puberty is often referred to as being a “late bloomer.” Cortisol: The “stress hormone”, which is produced by the adrenal glands.