Deep Tendon Reflexes (DTR): Aka Muscle Stretch Reflex (MSR)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Focusing on the Re-Emergence of Primitive Reflexes Following Acquired Brain Injuries
33 Focusing on The Re-Emergence of Primitive Reflexes Following Acquired Brain Injuries Resiliency Through Reconnections - Reflex Integration Following Brain Injury Alex Andrich, OD, FCOVD Scottsdale, Arizona Patti Andrich, MA, OTR/L, COVT, CINPP September 19, 2019 Alex Andrich, OD, FCOVD Patti Andrich, MA, OTR/L, COVT, CINPP © 2019 Sensory Focus No Pictures or Videos of Patients The contents of this presentation are the property of Sensory Focus / The VISION Development Team and may not be reproduced or shared in any format without express written permission. Disclosure: BINOVI The patients shown today have given us permission to use their pictures and videos for educational purposes only. They would not want their images/videos distributed or shared. We are not receiving any financial compensation for mentioning any other device, equipment, or services that are mentioned during this presentation. Objectives – Advanced Course Objectives Detail what primitive reflexes (PR) are Learn how to effectively screen for the presence of PRs Why they re-emerge following a brain injury Learn how to reintegrate these reflexes to improve patient How they affect sensory-motor integration outcomes How integration techniques can be used in the treatment Current research regarding PR integration and brain of brain injuries injuries will be highlighted Cases will be presented Pioneers to Present Day Leaders Getting Back to Life After Brain Injury (BI) Descartes (1596-1650) What is Vision? Neuro-Optometric Testing Vision writes spatial equations -

Improvement and Neuroplasticity After Combined Rehabilitation to Forced Grasping
Hindawi Case Reports in Neurological Medicine Volume 2017, Article ID 1028390, 7 pages https://doi.org/10.1155/2017/1028390 Case Report Improvement and Neuroplasticity after Combined Rehabilitation to Forced Grasping Michiko Arima, Atsuko Ogata, Kazumi Kawahira, and Megumi Shimodozono Department of Rehabilitation and Physical Medicine, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan Correspondence should be addressed to Michiko Arima; [email protected] Received 20 September 2016; Revised 31 December 2016; Accepted 9 January 2017; Published 6 February 2017 Academic Editor: Pablo Mir Copyright © 2017 Michiko Arima et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The grasp reflex is a distressing symptom but the need to treat or suppress it has rarely been discussed in the literature. We report the case of a 17-year-old man who had suffered cerebral infarction of the right putamen and temporal lobe 10 years previously. Forced grasping of the hemiparetic left upper limb was improved after a unique combined treatment. Botulinum toxin typeA (BTX-A) was first injected into the left biceps, wrist flexor muscles, and finger flexor muscles. Forced grasping was reduced along with spasticity of the upper limb. In addition, repetitive facilitative exercise and object-related training were performed under low-amplitude continuous neuromuscular electrical stimulation. Since this 2-week treatment improved upper limb function, we compared brain activities, as measured by near-infrared spectroscopy during finger pinching, before and after the combined treatment. -

The Grasp Reflex and Moro Reflex in Infants: Hierarchy of Primitive
Hindawi Publishing Corporation International Journal of Pediatrics Volume 2012, Article ID 191562, 10 pages doi:10.1155/2012/191562 Review Article The Grasp Reflex and Moro Reflex in Infants: Hierarchy of Primitive Reflex Responses Yasuyuki Futagi, Yasuhisa Toribe, and Yasuhiro Suzuki Department of Pediatric Neurology, Osaka Medical Center and Research Institute for Maternal and Child Health, 840 Murodo-cho, Izumi, Osaka 594-1101, Japan Correspondence should be addressed to Yasuyuki Futagi, [email protected] Received 27 October 2011; Accepted 30 March 2012 Academic Editor: Sheffali Gulati Copyright © 2012 Yasuyuki Futagi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The plantar grasp reflex is of great clinical significance, especially in terms of the detection of spasticity. The palmar grasp reflex also has diagnostic significance. This grasp reflex of the hands and feet is mediated by a spinal reflex mechanism, which appears to be under the regulatory control of nonprimary motor areas through the spinal interneurons. This reflex in human infants can be regarded as a rudiment of phylogenetic function. The absence of the Moro reflex during the neonatal period and early infancy is highly diagnostic, indicating a variety of compromised conditions. The center of the reflex is probably in the lower region of the pons to the medulla. The phylogenetic meaning of the reflex remains unclear. However, the hierarchical interrelation among these primitive reflexes seems to be essential for the arboreal life of monkey newborns, and the possible role of the Moro reflex in these newborns was discussed in relation to the interrelationship. -

Level Diagnosis of Cervical Compressive Myelopathy: Signs, Symptoms, and Lesions Levels
Elmer Press Original Article J Neurol Res • 2013;3(5):135-141 Level Diagnosis of Cervical Compressive Myelopathy: Signs, Symptoms, and Lesions Levels Naoki Kasahata ficult to accurately localize the lesion before radiographic Abstract diagnosis. However, neurological level diagnosis of spinal cord is important for accurate lesion-specific level diagnosis, Background: To elucidate signs and symptoms corresponding to patients’ treatment, avoiding diagnostic error, differential di- each vertebral level for level-specific diagnoses. agnosis, and especially for accurate level diagnosis of other nonsurgical myelopathies. Moreover, level diagnosis should Methods: We studied 106 patients with cervical compressive my- be considered from multiple viewpoints. Therefore, we in- elopathy. Patients who showed a single compressive site on mag- tend to make level diagnosis of myelopathy more accurate. netic resonance imaging (MRI) were selected, and signs, symp- Previously, lesion-specific level diagnoses by determin- toms, and the levels of the MRI lesions were studied. ing a sensory disturbance area or location of numbness in Results: Five of 12 patients (41.7%) with C4-5 intervertebral level the hands had the highest accuracy [1, 2]. Previous stud- lesions showed decreased or absent biceps and brachioradialis re- ies reported that C3-4 intervertebral level lesions showed flexes, while 4 of these patients (33.3%) showed generalized hyper- increased or decreased biceps reflexes, deltoid weakness, reflexia. In comparison, 5 of 24 patients (20.8%) with C5-6 inter- and sensory disturbance of arms or forearms [1, 3, 4], while vertebral level lesions showed decreased or absent triceps reflexes; C4-5 intervertebral level lesions showed decreased biceps however, 9 of these patients (37.5%) showed decreased or absent reflexes, biceps weakness, and sensory disturbance of hands biceps and brachioradialis reflexes. -

What's the Connection?
WHAT’S THE CONNECTION? Sharon Winter Lake Washington High School Directions for Teachers 12033 NE 80th Street Kirkland, WA 98033 SYNOPSIS Students elicit and observe reflex responses and distinguish between types STUDENT PRIOR KNOWL- of reflexes. They then design and conduct experiments to learn more about EDGE reflexes and their control by the nervous system. Before participating in this LEVEL activity students should be able to: Exploration, Concept/Term Introduction Phases ■ Describe the parts of a Application Phase neuron and explain their functions. ■ Distinguish between sensory and motor neurons. Getting Ready ■ Describe briefly the See sidebars for additional information regarding preparation of this lab. organization of the nervous system. Directions for Setting Up the Lab General: INTEGRATION Into the Biology Curriculum ■ Make an “X” on the chalkboard for the teacher-led introduction. ■ Health ■ Photocopy the Directions for Students pages. ■ Biology I, II ■ Human Anatomy and Teacher Background Physiology A reflex is an involuntary neural response to a specific sensory stimulus ■ AP Biology that threatens the survival or homeostatic state of an organism. Reflexes Across the Curriculum exist in the most primitive of species, usually with a protective function for ■ Mathematics animals when they encounter external and internal stimuli. A primitive ■ Physics ■ example of this protective reflex is the gill withdrawal reflex of the sea slug Psychology Aplysia. In humans and other vertebrates, protective reflexes have been OBJECTIVES maintained and expanded in number. Examples are the gag reflex that At the end of this activity, occurs when objects touch the sides students will be able to: or the back of the throat, and the carotid sinus reflex that restores blood ■ Identify common reflexes pressure to normal when baroreceptors detect an increase in blood pressure. -

Motor Neuron Disease (Amyotrophic Lateral Sclerosis) Arising from Longstanding Primary Lateral Sclerosis
74274ournal ofNeurology, Neurosurgery, and Psychiatry 1995;58:742-744 SHORT REPORT J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.58.6.742 on 1 June 1995. Downloaded from Motor neuron disease (amyotrophic lateral sclerosis) arising from longstanding primary lateral sclerosis R P M Bruyn, J H T M Koelman, D Troost,J M B V de Jong Abstract family history was negative and consanguinity Three men were initially diagnosed as was excluded. Examination disclosed slight having primary lateral sclerosis (PLS), weakness of the left thigh muscles, mild spas- but eventually developed amyotrophic ticity of the legs, knee and ankle cloni, and lateral sclerosis (ALS) after 7-5, 9, and at extensor plantars. Blood chemistry, CSF, and least 27 years. Non-familial ALS and EMG were unremarkable. Magnetic reso- PLS might be different manifestations of nance imaging of the cervical spine was nor- a single disease or constitute completely mal and PLS was diagnosed. Micturition distinct entities. The clinical diagnosis of urgency began in 1987. Examination in 1988 PLS predicts a median survival that is showed definite spastic paraparesis and mild four to five times longer than in ALS. proximal weakness of the legs. Sural and tib- ial nerve somatosensory evoked potentials (J Neurol Neurosurg Psychiatry 1995;58:742-744) (SSEPs) were bilaterally absent and delayed respectively. Visual and brainstem auditory evoked potentials (VEPs, BAEPs), and brain Keywords: amyotrophic lateral sclerosis; primary MRI were normal. By 1990, walking had lateral sclerosis become troublesome and dysarthria was pre- sent; the calves showed fasciculation and Primary lateral sclerosis (PLS), defined as some atrophy. -
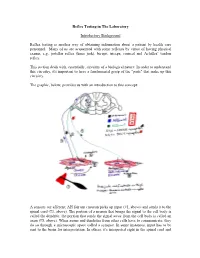
Reflex Testing in the Laboratory
Reflex Testing in The Laboratory Introductory Background Reflex testing is another way of obtaining information about a patient by health care personnel. Many of us are acquainted with some reflexes by virtue of having physical exams, e.g., patellar reflex (knee jerk), biceps, triceps, corneal and Achilles’ tendon reflex. This section deals with, essentially, circuitry of a biological nature. In order to understand this circuitry, it's important to have a fundamental grasp of the "parts" that make up this circuitry. The graphic, below, provides us with an introduction to this concept: A sensory (or afferent; AH fair unt) neuron picks up input (#1, above) and sends it to the spinal cord (#3, above). The portion of a neuron that brings the signal to the cell body is called the dendrite; the portion that sends the signal away from the cell body is called an axon (#5, above). When axons and dendrites from other cells have to communicate, they do so through a microscopic space called a synapse. In some instances, input has to be sent to the brain for interpretation. In others, it's interpreted right in the spinal cord and signals are sent out (motor or efferent; EE fair unt) to the effector organ. In simple stretch reflexes, only two neurons are involved: sensory and motor, graphic, above. In this figure, a stretch reflex is illustrated. The way it works is in this manner: 1) a tendon is stimulated (in this illustration by a reflex hammer), 2) the spindle (blue coil in the diagram) detects this stimulus and sends the input to the cord, 3) the information crosses one synapse (mono-synaptic) to a motor neuron that sends output to the spindle (green coil in diagram) and the muscle contracts. -

The Nervous System Reflexes Spinal Reflexes Reflex Arc the Stretch
1/17/2016 Reflexes • Rapid, involuntary, predictable motor response to a stimulus The Nervous System Spinal Reflexes Spinal Reflexes Reflex Arc • Spinal somatic reflexes • Components of a reflex arc – Integration center is in the spinal cord 1. Receptor—site of stimulus action – Effectors are skeletal muscle 2. Sensory neuron—transmits afferent impulses to the CNS • Testing of somatic reflexes is important clinically 3. Synapses in gray matter—either monosynaptic or to assess the condition of the nervous system polysynaptic region within the CNS 4. Motor neuron—conducts efferent impulses away from cord • Identical stimulus should always elicit the same 5. Effector—muscle fiber or gland cell that responds to response stereotyped reflex the efferent impulses by contracting or secreting Stimulus The Stretch Reflex Skin • Monosynaptic reflex – 2 neurons (sensory and motor), 1 synapse 1 Receptor Interneuron • Muscle spindles 2 Sensory neuron – Sensory receptors in belly of muscle 3 Integration center – Detects changes in length of muscle 4 Motor neuron • Muscle is stretched, reflex reverses the stretch 5 Effector • Important for coordination, maintenance of posture, keeps muscles from over stretching Spinal cord (in cross section) Figure 13.14 1 1/17/2016 Secondary sensory The patellar (knee-jerk) reflex—a specific example of a stretch reflex Efferent (motor) endings (type II fiber – fiber to muscle spindle senses when muscle 2 is still) Quadriceps 3a (extensors) 3b 3b ααα Efferent (motor) 1 Primary sensory fiber to extrafusal Patella endings (type Ia Muscle Spinal cord muscle fibers spindle Fiber – senses (L 2–L4) stretching) Extrafusal muscle 1 Tapping the patellar ligament excites fiber Hamstrings Patellar muscle spindles in the quadriceps. -

The Serotonin Syndrome
The new england journal of medicine review article current concepts The Serotonin Syndrome Edward W. Boyer, M.D., Ph.D., and Michael Shannon, M.D., M.P.H. From the Division of Medical Toxicology, he serotonin syndrome is a potentially life-threatening ad- Department of Emergency Medicine, verse drug reaction that results from therapeutic drug use, intentional self-poi- University of Massachusetts, Worcester t (E.W.B.); and the Program in Medical Tox- soning, or inadvertent interactions between drugs. Three features of the sero- icology, Division of Emergency Medicine, tonin syndrome are critical to an understanding of the disorder. First, the serotonin Children’s Hospital, Boston (E.W.B., M.S.). syndrome is not an idiopathic drug reaction; it is a predictable consequence of excess Address reprint requests to Dr. Boyer at IC Smith Bldg., Children’s Hospital, 300 serotonergic agonism of central nervous system (CNS) receptors and peripheral sero- 1,2 Longwood Ave., Boston, MA 02115, or at tonergic receptors. Second, excess serotonin produces a spectrum of clinical find- [email protected]. edu. ings.3 Third, clinical manifestations of the serotonin syndrome range from barely per- This article (10.1056/NEJMra041867) was ceptible to lethal. The death of an 18-year-old patient named Libby Zion in New York updated on October 21, 2009 at NEJM.org. City more than 20 years ago, which resulted from coadminstration of meperidine and phenelzine, remains the most widely recognized and dramatic example of this prevent- N Engl J Med 2005;352:1112-20. 4 Copyright © 2005 Massachusetts Medical Society. able condition. -

The Clinical Approach to Movement Disorders Wilson F
REVIEWS The clinical approach to movement disorders Wilson F. Abdo, Bart P. C. van de Warrenburg, David J. Burn, Niall P. Quinn and Bastiaan R. Bloem Abstract | Movement disorders are commonly encountered in the clinic. In this Review, aimed at trainees and general neurologists, we provide a practical step-by-step approach to help clinicians in their ‘pattern recognition’ of movement disorders, as part of a process that ultimately leads to the diagnosis. The key to success is establishing the phenomenology of the clinical syndrome, which is determined from the specific combination of the dominant movement disorder, other abnormal movements in patients presenting with a mixed movement disorder, and a set of associated neurological and non-neurological abnormalities. Definition of the clinical syndrome in this manner should, in turn, result in a differential diagnosis. Sometimes, simple pattern recognition will suffice and lead directly to the diagnosis, but often ancillary investigations, guided by the dominant movement disorder, are required. We illustrate this diagnostic process for the most common types of movement disorder, namely, akinetic –rigid syndromes and the various types of hyperkinetic disorders (myoclonus, chorea, tics, dystonia and tremor). Abdo, W. F. et al. Nat. Rev. Neurol. 6, 29–37 (2010); doi:10.1038/nrneurol.2009.196 1 Continuing Medical Education online 85 years. The prevalence of essential tremor—the most common form of tremor—is 4% in people aged over This activity has been planned and implemented in accordance 40 years, increasing to 14% in people over 65 years of with the Essential Areas and policies of the Accreditation Council age.2,3 The prevalence of tics in school-age children and for Continuing Medical Education through the joint sponsorship of 4 MedscapeCME and Nature Publishing Group. -

The Phenomenon of Multiple Stretch Reflexes
Henry Ford Hospital Medical Journal Volume 34 Number 1 Article 6 3-1986 The Phenomenon of Multiple Stretch Reflexes Robert D. Teasdall Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Teasdall, Robert D. (1986) "The Phenomenon of Multiple Stretch Reflexes," Henry Ford Hospital Medical Journal : Vol. 34 : No. 1 , 31-36. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol34/iss1/6 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. The Phenomenon of Multiple Stretch Reflexes Robert D. Teasdall, MD* Multiple stretch reflexes occur in muscles adjacent to or remote from the tap. The response may be ipsilateral or bilateral. These reflexes are encountered not only in normal subjects with brisk stretch reflexes but particularly in patients with lesions of the upper motor neuron. The concussion obtained by the blow is conducted along bone to muscle. Muscle spindles are stimulated, and in this manner independent stretch reflexes are produced in these muscles. This mechanism is responsible for the phenomenon of multiple stretch reflexes. The thorax and pelvis play important roles in the contralateral responses by transmitting these mechanical events across the midline. (Henry FordHosp Med J 1986;34:31-6) ontraction of muscles remote from the site of f)ercussion is Head and neck Cencountered in patients with brisk stretch reflexes. -

Spinal Reflexes
Spinal Reflexes Lu Chen, Ph.D. MCB, UC Berkeley 1 Simple reflexes such as stretch reflex require coordinated contraction and relaxation of different muscle groups Categories of Muscle Based on Direction of Motion Flexors Æ reduce the angle of joints Extensors Æ increase the angle of joints Categories of Muscle Based on Movement Agonist Æmuscle that serves to move the joint in the same direction as the studied muscle Antagonist Æ muscle that moves the joint in the opposite direction 2 1 Muscle Spindles •Small encapsulated sensory receptors that have a Intrafusal muscle spindle-like shape and are located within the fibers fleshy part of the muscle •In parallel with the muscle fibers capsule •Does not contribute to the overall contractile Sensory force endings •Mechanoreceptors are activated by stretch of the central region Afferent axons •Due to stretch of the whole muscle Efferent axons (including intrafusal f.) •Due to contraction of the polar regions of Gamma motor the intrafusal fibers endings 3 Muscle Spindles Organization 2 kinds of intrafusal muscle fibers •Nuclear bag fibers (2-3) •Dynamic •Static •Nuclear chain fibers (~5) •Static 2 types of sensory fibers •Ia (primary) - central region of all intrafusal fibers •II (secondary) - adjacent to the central region of static nuclear bag fibers and nuclear chain fibers Intrafusal fibers stretched Sensory ending stretched, (loading the spindle) increase firing Muscle fibers lengthens Sensory ending stretched, (stretched) increase firing Spindle unloaded, Muscle fiber shortens decrease firing 4 2 Muscle Spindles Organization Gamma motor neurons innervate the intrafusal muscle fibers. Activation of Shortening of the polar regions gamma neurons of the intrafusal fibers Stretches the noncontractile Increase firing of the center regions sensory endings Therefore, the gamma motor neurons provide a mechanism for adjusting the sensitivity of the muscle spindles.