Cerebellar Disease Focussed Examination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detection of Focal Cerebral Hemisphere Lesions Using the Neurological Examination N E Anderson, D F Mason, J N Fink, P S Bergin, a J Charleston, G D Gamble
545 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.043679 on 16 March 2005. Downloaded from PAPER Detection of focal cerebral hemisphere lesions using the neurological examination N E Anderson, D F Mason, J N Fink, P S Bergin, A J Charleston, G D Gamble ............................................................................................................................... J Neurol Neurosurg Psychiatry 2005;76:545–549. doi: 10.1136/jnnp.2004.043679 Objective: To determine the sensitivity and specificity of clinical tests for detecting focal lesions in a prospective blinded study. Methods: 46 patients with a focal cerebral hemisphere lesion without obvious focal signs and 19 controls with normal imaging were examined using a battery of clinical tests. Examiners were blinded to the diagnosis. The sensitivity, specificity, and positive and negative predictive values of each test were measured. See end of article for authors’ affiliations Results: The upper limb tests with the greatest sensitivities for detecting a focal lesion were finger rolling ....................... (sensitivity 0.33 (95% confidence interval, 0.21 to 0.47)), assessment of power (0.30 (0.19 to 0.45)), rapid alternating movements (0.30 (0.19 to 0.45)), forearm rolling (0.24 (0.14 to 0.38)), and pronator Correspondence to: Dr Neil Anderson, drift (0.22 (0.12 to 0.36)). All these tests had a specificity of 1.00 (0.83 to 1.00). This combination of tests Department of Neurology, detected an abnormality in 50% of the patients with a focal lesion. In the lower limbs, assessment of power Auckland Hospital, Private was the most sensitive test (sensitivity 0.20 (0.11 to 0.33)). -

A Neurological Examination
THE 3 MINUTE NEUROLOGICAL EXAMINATION DEMYSTIFIED Faculty: W.J. Oczkowski MD, FRCPC Professor and Academic Head, Division of Neurology, Department of Medicine, McMaster University Stroke Neurologist, Hamilton Health Sciences Relationships with commercial interests: ► Not Applicable Potential for conflict(s) of interest: ► Not Applicable Mitigating Potential Bias ► All the recommendations involving clinical medicine are based on evidence that is accepted within the profession. ► All scientific research referred to, reported, or used is in the support or justification of patient care. ► Recommendations conform to the generally accepted standards. ► Independent content validation. ► The presentation will mitigate potential bias by ensuring that data and recommendations are presented in a fair and balanced way. ► Potential bias will be mitigated by presenting a full range of products that can be used in this therapeutic area. ► Information of the history, development, funding, and the sponsoring organizations of the disclosure presented will be discussed. Objectives ► Overview of neurological assessment . It’s all about stroke! . It’s all about the chief complaint and history. ► Overview: . 3 types of clinical exams . Neurological signs . Neurological localization o Pathognomonic signs o Upper versus lower motor neuron signs ► Cases and practice Bill ► 72 year old male . Hypertension . Smoker ► Stroke call: dizzy, facial droop, slurred speech ► Neurological Exam: . Ptosis and miosis on left . Numb left face . Left palatal weakness . Dysarthria . Ataxic left arm and left leg . Numb right arm and leg NIH Stroke Scale Score ► LOC: a,b,c_________________ 0 ► Best gaze__________________ 0 0 ► Visual fields________________ 0 ► Facial palsy________________ 0 ► Motor arm and leg__________ -Left Ptosis 2 -Left miosis ► Limb ataxia________________ -Weakness of 1 ► Sensory_______________________ left palate ► Best Language______________ 0 1 ► Dysarthria_________________ 0 ► Extinction and inattention____ - . -

THE NEUROLOGY Exam & Clinical Pearls
THE NEUROLOGY Exam & Clinical Pearls Gaye McCafferty, RN, MS, NP-BC, MSCS, SCRN NPANYS-SPHP Education Day Troy, New York April 7, 2018 Objectives I. Describe the core elements of the neurology exam II. List clinical pearls of the neuro exam Neurology Exam . General Physical Exam . Mental Status . Cranial Nerves . Motor Exam . Reflex Examination . Sensory Exam . Coordination . Gait and Station 1 General Systemic Physical Exam Head Trauma Dysmorphism Neck Tone Thyromegaly Bruits MSOffice1 General Systemic Physical Exam .Cardiovascular . Heart rate, rhythm, murmur; peripheral pulses, JVD .Pulmonary . Breathing pattern, cyanosis, Mallampati airway .General Appearance Hygiene, grooming, weight (signs of self neglect) .Funduscopic Exam Mental Status Level of Consciousness . Awake . Drowsy . Somnolent . Comatose 2 Slide 5 MSOffice1 , 6/14/2009 Orientation & Attention . Orientation . Time . Place . Person Orientation & Attention . Attention . Digit Span-have the patient repeat a series of numbers, start with 3 or 4 in a series and increase until the patient makes several mistakes. Then explain that you want the numbers backwards. Normal-seven forward, five backward Hint; use parts of telephone numbers you know Memory Immediate recall and attention Tell the patient you want him to remember a name and address – Jim Green – 20 Woodlawn Road, Chicago Note how many errors are made in repeating it and how many times you have to repeat it before it is repeated correctly. Normal: Immediate registration 3 Memory . Short-term memory . About 5 minutes after asking the patient to remember the name and address, ask him to repeat it. Long –term memory . Test factual knowledge . Dates of WWII . Name a president who was shot dead Memory Mini-Mental State Exam – 30 items Mini-Cog – Rapid Screen for Cognitive Impairment – A Composite of 3 item recall and clock drawing – Takes about 5 minutes to administer Mini-Cog Mini-Cog Recall 0 Recall 1-2 Recall 3 Demented Non-demented Abnormal Clock Normal Clock Demented Non-demented 4 Memory . -

Cerebellar Examination 1. General Inspection As Above 2. Posture
Cerebellar Examination 1. General Inspection as above 2. Posture: - Whilst the child is sitting, get him/her to lift his feet from the ground with arms crossed (Truncal ataxia) - Get the child to stand up and maintain position with feet together and eyes opened. And then with eyes closed (Rhomberg ’s test). If the child is ataxic and unsteady with eyes closed (Rhomberg ’s test positive), then the problem is likely to be sensory ataxia, rather than cerebellar ataxia. 3. Gait - Get patient to walk (broad-based ataxic gait, falls towards the side of the lesion) , then ask him to STOP, turn back and do - Heel to toe walk 4. Face - Eyes : H test for extraocular muscles and pause at lateral gaze – horizontal nystagmus, towards the side of the lesion (lateral cerebellar lesion) - Speech: Ask the child question / ask him to read/ for older child, ask him to say baby hippotamus, West register street/ british constitution (staccato speech/ scanning dysarthria) 5. Upper limbs -Pronator drift – ask patient to hold his arms out with his palm facing upwards and his eyes closed : Pronator drift – weakness ; Upward drift – cerebellar lesion -Rebound test- whilst patients arms are held out, push his wrist down quickly (Holmes’ rebound phenomenon – over correction of passive displacement of limb) -Hypotonia - Rapid palm test (Dysdiadoschokinesia) -Finger-nose test (Dysmetria) 6. Lower limbs - Hypotonia - Reflex: pendular reflex, best seen when patients limb left hanging in the air - Heel-shin test To complete the examination, I would -check the fundi for papilloedema (space occupying lesion) - perform a full neurological examination Causes of cerebellar lesions: 1. -

Tests of Motor Function in Patients Suspected of Having Mild Unilateral Cerebral Lesions
ORIGINAL ARTICLE Tests of Motor Function in Patients Suspected of Having Mild Unilateral Cerebral Lesions Jeanne S. Teitelbaum, Michael Eliasziw, Michael Garner ABSTRACT: Objective: Though various textbooks describe clinical manoeuvres that help detect subtle motor deficits, their sensitivi- ty, specificity and predictive values have not been determined. We investigated the sensitivity, specificity and predictive values of var- ious manoeuvres in order to determine the most sensitive and reliable test or combination thereof. Methods: Straight arm raising (Barré), pronator drift, Mingazzini’s manoeuvre, finger tap, forearm roll, segmental strength and deep tendon reflexes were tested in 170 patients with (86) and without (84) a proven lesion in the motor areas confirmed by computed tomography. Results: Segmental motor strength had good specificity (97.5%) but poor sensitivity (38.9%) and negative predictive value (NPV) (58.7%). The forearm roll had a similar profile. Finger tap had a sensitivity of 73.3% and a specificity of 87.5%. Barré and pronator testing had a sensitivity and specificity of 92.2% and 90.0% respectively. Hyperreflexia had a sensitivity of 68.9% and a specificity of 87.5%. An abnormality of pronator, reflex- es or finger tap had a sensitivity of 97%, and when these three tests were positive, specificity was 97%. When all six tests were posi- tive, the positive predictive value was 100%, when all six tests were negative the NPV was 100%. Conclusion: The detailed segmen- tal examination has very good specificity for detecting motor deficits, but the sensitivity and NPV are unacceptably low. Pronator drift with finger tap and reflexes is the most reliable and time-effective combination of tests for the detection of subtle motor lesions, and could replace the segmental motor examination as a screening for motor lesions. -

124 Epochs of Anosmia and Ageusia in Multiple Sclerosis
78 ABSTRACTS 2 Director, Head of Corporate Medical Affairs, H. 29.0 for patients with anxious distress in the adjunctive Lundbeck A/S, Valby, Denmark brexpiprazole (n = 462) group and 29.1 in the placebo 3 Director, Global Medical Affairs, Otsuka (n = 327) group; while those with anxious depression Pharmaceutical Development & Commercialization, were 28.9 (brexpiprazole; n = 384) and 28.6 (placebo; Inc., Princeton, NJ, USA n = 282). Compared to those receiving placebo, patients 4 Senior Director, Biostatistics, Otsuka Pharmaceutical with both anxious distress and anxious depression who Development & Commercialization, Inc., Princeton, received adjunctive brexpiprazole showed a greater NJ, USA 5 Senior Director, Global Medical Affairs, Otsuka improvement in MADRS total score (LS mean difference = = Pharmaceutical Development & Commercialization, -2.38, p 0.0001 and -1.68, p 0.012, respectively). Inc., Princeton, NJ, USA These improvements, compared to placebo, were similar to those in patients who had not met the criteria for ABSTRACT: Study objectives: Symptoms of anxiety are anxious distress (-1.40, p = 0.023) or anxious depression prevalent in Major Depressive Disorder (MDD) and are (-2.17, p < 0.001). associated with greater illness severity, suicidality, impaired functioning and poor response to antidepres- CONCLUSION: Adjunctive brexpiprazole may be effica- sant treatment (ADT). In MDD, anxiety symptoms can be cious in reducing depressive symptoms both in patients assessed as ‘anxious distress’ (new DSM-5 specifier) or with or without symptoms of anxiety. ‘anxious depression’ (score ≥7 on the HAM-D anxiety/ FUNDING ACKNOWLEDGEMENTS: The studies were funded somatization factor). Brexpiprazole is a serotonin– by H. Lundbeck A/S and Otsuka Pharmaceutical Devel- dopamine activity modulator that is a partial agonist at opment & Commercialization, Inc. -

Validity of the “Drift Without Pronation” Sign in Conversion Disorder Corinna Daum and Selma Aybek*
Daum and Aybek BMC Neurology 2013, 13:31 http://www.biomedcentral.com/1471-2377/13/31 RESEARCH ARTICLE Open Access Validity of the “Drift without pronation” sign in conversion disorder Corinna Daum and Selma Aybek* Abstract Background: Conversion disorder (CD) is a psychiatric disorder, yet the diagnosis cannot be established without the expertise of a neurologist, as distinguishing a functional from an organic symptom relies on careful bedside examination. Joseph Babinski considered the absence of pronator drift as a ‘positive sign’ for hysterical paresis but the validity of this sign has never been evaluated. The aim of this study was to examine the sensitivity and specificity of the “drift without pronation” sign. Methods: Twenty-six patients with unilateral functional upper limb paresis diagnosed with CD (DSM-IV) and a control group of 28 patients with an organic neurological condition were consecutively included. The arm stabilisation test was performed with arms stretched out in full supination, fingers adducted, eyes closed for 10 seconds. A positive “drift without pronation” sign was defined by the presence of a downward drift without pronation. Results: All CD subjects (100%) displayed a positive sign when only 7.1% of organic subjects did (Fisher’s p < 0.001). The sign yielded a sensitivity of 100% (95% CI:84%-100%) and a specificity of 93% (95% CI:76%-98%). Conclusion: The observation of a “drift without pronation” sign is specific for Conversion Disorder and can be of help in making a quick distinction between organic and functional paresis at the bedside. Keywords: Conversion disorder, Pronator drift, Arm paresis, Functional symptom Background specificity has been a recent focus of clinical research. -

A Dictionary of Neurological Signs.Pdf
A DICTIONARY OF NEUROLOGICAL SIGNS THIRD EDITION A DICTIONARY OF NEUROLOGICAL SIGNS THIRD EDITION A.J. LARNER MA, MD, MRCP (UK), DHMSA Consultant Neurologist Walton Centre for Neurology and Neurosurgery, Liverpool Honorary Lecturer in Neuroscience, University of Liverpool Society of Apothecaries’ Honorary Lecturer in the History of Medicine, University of Liverpool Liverpool, U.K. 123 Andrew J. Larner MA MD MRCP (UK) DHMSA Walton Centre for Neurology & Neurosurgery Lower Lane L9 7LJ Liverpool, UK ISBN 978-1-4419-7094-7 e-ISBN 978-1-4419-7095-4 DOI 10.1007/978-1-4419-7095-4 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010937226 © Springer Science+Business Media, LLC 2001, 2006, 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. -

The Advanced Neurologic Exam Principles and Practice Robert T
The Advanced Neurologic Exam Principles and Practice Robert T. Naismith, MD Neurology Clerkship Director 7/2018 Objectives • Students will perform a: • Detailed and comprehensive neurologic examination, • With optimized positioning and phrasing, • Based upon hypotheses from history and prior observations, • To build evidence for a finding or system to be reliably present/absent, • By interconnecting historical and examination components, • With an appreciation of sensitivity/specificity, subjectivity/objectivity, • While ensuring the big picture makes sense. Transferability of Hypothesis Generation • Clinical and scientific approach to: • Bedside Diagnosis in Medical and Surgical Specialties • Image Review and Interpretation • Operations and Procedures more Efficient with Fewer Complications • Histopathology Review and Interpretation • Critical Thinking and Deductive Reasoning • Developing and Going through Your Process • Searching for Clues • Maintain an Open and Nimble Mind • Building a Case, Recognizing Pros/Cons, Typical/Atypical • Determining Best Approach to Proceed Approach • Take your time, there is no need to do a 5 minute neuro exam • The history should include functional elements to preview the exam • Bring your hypotheses to the exam, so you can scrutinize your findings • Make a cheat sheet/scoring sheet • After the history, pause: • Where do I localize their symptoms? • What diseases occur in those locations? • What am I expecting to find on exam? • UMN, LMN, motor, cerebellar, sensory, extrapyramidal, etc • After each section of the exam, document and pause: • Does this make sense with regards to the big picture? • Am I confident in the findings I elicited? Do any need repeating? • Any additional maneuvers I should consider based upon localization and differential, or indeterminant/inconsistent findings? Increasing Reliability • Patient positioning • Sitting vs. -

Medical Student Observation Guide
Figure e-1. Observation Guide for Students MEDICAL STUDENT OBSERVATION GUIDE BEDSIDE SKILLS MODELING FOR THIRD YEAR MEDICAL STUDENTS DEPARTMENT OF NEUROLOGY UNIVERSITY OF ROCHESTER Modeling: A group of faculty neurologists have been selected to provide you with a formal modeling experience. During the first week of the clerkship, you will be assigned to a specific faculty neurologist preceptor, and will be given the date, time, and location of the patient encounter. All modeling encounters will take place during the physician’s regularly scheduled clinic. As you are meant to see a full history and neurological exam modeled by an attending physician, it will be a new patient encounter that should be carried out directly by the attending and not a resident or fellow. Please plan on arriving at the encounter 5 minutes early. After the case, you will have 5 minutes to ask questions about the encounter, and the physician preceptor should highlight important points. You will fill out the below observation questionnaire during the experience. The guide will list all of the aspects of the history and neurologic exam that you may be observing. Please note that all of these items may not be directly tested in each encounter as many neurologists will do focused examinations based on the chief complaint. Please list general observations and/or questions you have that may arise during the encounter. Also, think about what was done during the visit that was surprising to you or different from what you expected. When participating in the observation experience, you should focus on the technique utilized by your preceptor in obtaining various aspects of the history and performing various aspects of the exam. -
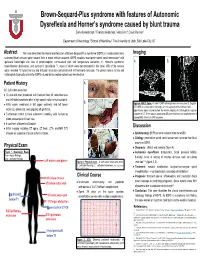
Brown-Sequard-Plus Syndrome with Features of Autonomic Dysreflexia
Brown-Sequard-Plus syndrome with features of Autonomic Dysreflexia and Horner’s syndrome caused by blunt trauma 'DQLHOD$QGHUVRQ7KRPDV$QGHUVRQ $OH[/LQQ'DYLG5HQQHU 'HSDUWPHQWRI1HXURORJ\6FKRRORI0HGLFLQH 7KH8QLYHUVLW\RI8WDK6DOW/DNH&LW\87 Abstract 7KLVFDVHGHVFULEHVWKHFOLQLFDOPDQLIHVWDWLRQRI%URZQ6HTXDUG3OXVV\QGURPH %636 LQDPDOHSDWLHQWZKR Imaging VXVWDLQHG EOXQW FHUYLFDO VSLQH WUDXPD IURP D PRWRU YHKLFOH DFFLGHQW %636 LQYROYHV LQFRPSOHWH VSLQDO FRUG KHPLVHFWLRQ ZLWK LSVLODWHUDO KHPLSOHJLD DQG ORVV RI SURSULRFHSWLRQ FRQWUDODWHUDO SDLQ DQG WHPSHUDWXUH VHQVDWLRQ +RUQHU¶V V\QGURPH $ % ERZHOEODGGHU G\VIXQFWLRQ DQG DXWRQRPLF G\VUHIOH[LD PDQ\ RI ZKLFK ZHUH GHPRQVWUDWHG LQ WKLV FDVH 05, RI WKH FHUYLFDO VSLQH UHYHDOHG 7 K\SHULQWHQVLW\ DQG GLIIXVLRQ UHVWULFWLRQ FRQVLVWHQW ZLWK OHIW KHPLFRUG FRQWXVLRQ 7KH SDWLHQW PHHWV FOLQLFDO DQG UDGLRORJLFDO GLDJQRVWLF FULWHULD IRU %636 FDXVHG E\ LQFRPSOHWH VSLQDO FRUG KHPLVHFWLRQ Patient History $ && /HIWVLGHG ZHDNQHVV Ɣ \HDUROG PDQ SUHVHQWHG ZLWK WUDQVLHQW ORVV RI FRQVFLRXVQHVV DQG OHIWVLGHG ZHDNQHVV DIWHU D KLJK VSHHG PRWRU YHKLFOH DFFLGHQW % Ɣ ,QLWLDO H[DP ZHDNQHVV LQ OHIW XSSHU H[WUHPLW\ DQG OHIW ORZHU Figure 4: MRI C Spine. $$[LDO705,VKRZLQJKHPLFRUGFRQWXVLRQ%6DJLWWDO 67,505,RIFHUYLFDOVSLQHVKRZLQJDFPVHJPHQWRIWKHOHIWKHPLFRUG H[WUHPLW\ DQLVRFRULD DQG XSJRLQJ OHIW JUHDW WRH K\SHULQWHQVHVLJQDOH[WHQGLQJIURPWKHLQIHULRUHQGSODWHRI&WKURXJKWKHVXSHULRU Ɣ )XUWKHUPRUH QRWHG WR KDYH DXWRQRPLF LQVWDELOLW\ ZLWK IOXFWXDWLQJ HQGSODWHRI&7KHUHZDVDQDVVRFLDWHGGLIIXVLRQUHVWULFWLRQDQGVPDOOVHJPHQWRI EORRG SUHVVXUHV DQG KHDUW UDWH VXVFHSWLELOLW\ -

The Five-Minute Neurological Examination
The Five-Minute Neurological Examination Ralph F. Józefowicz, MD Mental Status •Cognition •Language •Naming Cranial Nerves •Visual fields •Pupils •Eye movements •Pursuits and saccades •Facial symmetry •Eye blinks •Lower cranial nerves Motor Examination •Adventitial movements •Pronator drift •External rotation of leg •Muscle tone •Functional strength testing Sensory Examination •Focus on patient’s symptoms •Don’t over-interpret •Thoracic sensory level •Touching nose with eyes closed •Romberg test Coordination •Ataxia •Cerebellar •Sensory disorders •Upper motor neuron lesion •Truncal stability Reflexes •Purely objective •Asymmetry •Ankle clonus •Babinski sign – don’t over-interpret Gait •Base •Stride •Arm swing •Turns •Symmetry Examination Order 1 •History •Mental status •Adventitial movements •Facial symmetry •Gait •Casual •Heel •Toe •Tandem Examination Order 2 •Truncal stability and Romberg test •Functional motor testing •Upper limbs •Lower limbs •Visual fields, pupils, eye movements Examination Order 3 •Motor examination •Pronator drift •Finger-to-nose testing with eyes closed •Motor tone •Hand grips •Reflexes •Muscle stretch reflexes •Babinski sign Cases Case 1 A 68-year-old woman with hypertension was brought to the emergency department by her friend because of dizziness, vertigo and difficulty walking, which she first noted when she awoke from a nap that evening. BP=200/130 mm Hg and P=76/min. She is examined lying on a gurney in the emergency department. There is minimal nystagmus with right gaze. Facial strength and sensation are