Rare Leaf Fossils of Monimiaceae and Atherospermataceae (Laurales) from Eocene Patagonian Rainforests and Their Biogeographic Significance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Susceptibility of Australian Plant Species to Phytophthora Ramorum1
GENERAL TECHNICAL REPORT PSW-GTR-229 Susceptibility of Australian Plant Species to 1 Phytophthora ramorum Kylie Ireland,2,3 Daniel Hüberli,3,4 Bernard Dell,3 Ian Smith,5 David Rizzo,6 and Giles Hardy3 Abstract Phytophthora ramorum is an invasive plant pathogen causing considerable and widespread damage in nurseries, gardens, and natural woodland ecosystems of the United States and Europe, and is classified as a Category 1 pest in Australia. It is of particular interest to Australian plant biosecurity as, like P. cinnamomi; it has the potential to become a major economic and ecological threat in areas with susceptible hosts and conducive climates. Research was undertaken in California to assess pathogenicity of P. ramorum on Australian native plants. Sixty-eight test species within 24 families were sourced from established gardens and arboretums. Foliar and branch susceptibility were tested using detached leaf and branch assays. The experiment was repeated to account for seasonality. Initial results indicate the majority of species tested were susceptible to varying degrees. Of particular interest are the high levels of variability within the eucalypts, low levels of susceptibility within the Pittosporaceae family, and a concerning number of latent or asymptomatic infections. Introduction Phytophthora ramorum, the cause of sudden oak death in California, is an invasive plant pathogen causing considerable and widespread damage in nurseries, gardens, and natural woodland ecosystems of the United States (U.S.) and Europe (Werres and others 2001, Rizzo and others 2002, Brasier and others 2004). Classified as a Category 1 pest in Australia (Plant Health Australia 2006), it is of particular concern to Australian plant biosecurity as, like P. -

Supplementary Information Of
Supplementary Information of Elevated CO2 and Global Greening during the early Miocene Tammo Reichgelt1,2, William J. D’Andrea1, Ailín del C. Valdivia-McCarthy1, Bethany R.S. Fox3, Jennifer M. Bannister4, John G. Conran5, William G. Lee6,7, Daphne E. Lee8 Correspondence to: Tammo Reichgelt ([email protected]) • Section S1: Morphotype Identification • Figure S1: Examples of cuticles of morphotypes A–K • Figure S2: Examples of cuticles of morphotypes L–V • Table S1: Measurements of stomatal density, pore length, guard cell width, leaf and air carobn isotopic composition of fossil leaves from Foulden Maar. • Table S2: Assimilation rate range of modern living relatives. • Table S3: Intrinsic water use efficiency, conductance to water, total annual carbon gain and length of the growing season. • Table S4: Output of reconstructed atmospheric carbon dioxide, carbon assimilation rates, leaf conductance to water, and intrinsic water use efficiency of Foulden Maar fossil leaves. • References for morphotype identification. Morphotype identification 18 distinct leaf morphotypes were identified based on microscopic anatomical features. Some leaf types were assigned to previously described species from surface exposures at Foulden Maar, others were tentatively assigned genera or families. Below are the leaf fossils assigned to different morphotypes, together with defining anatomical characteristics and justification of assignment to a specific plant group. Morphotype: A. Samples: (25) 9-09-50-1, 14-02-40-5, 17-91-70-1, 17-91-70-5, 17-91-70-7, 17-91-70-8, 18-19-99-1, 31- 19-10-1, 31-19-20-3, 31-19-20-4, 32-20-30-1, 32-20-60-1, 38-97-90-10, 40-93-80-4, 41-88-12-1, 41-88- 28-4, 41-88-28-7, 42-85-0-2, 56-31-20-1, 62-19-2-1, 63-19-95-3, 83-48-86-2 (Fig. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -
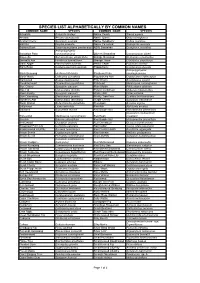
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

Doctorat De L'université De Toulouse
En vue de l’obt ention du DOCTORAT DE L’UNIVERSITÉ DE TOULOUSE Délivré par : Université Toulouse 3 Paul Sabatier (UT3 Paul Sabatier) Discipline ou spécialité : Ecologie, Biodiversité et Evolution Présentée et soutenue par : Joeri STRIJK le : 12 / 02 / 2010 Titre : Species diversification and differentiation in the Madagascar and Indian Ocean Islands Biodiversity Hotspot JURY Jérôme CHAVE, Directeur de Recherches CNRS Toulouse Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Frédéric MEDAIL, Professeur à l'Université Paul Cezanne Aix-Marseille Christophe THEBAUD, Professeur à l'Université Paul Sabatier Ecole doctorale : Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB) Unité de recherche : UMR 5174 CNRS-UPS Evolution & Diversité Biologique Directeur(s) de Thèse : Christophe THEBAUD Rapporteurs : Emmanuel DOUZERY, Professeur à l'Université de Montpellier II Porter LOWRY II, Curator Missouri Botanical Garden Contents. CONTENTS CHAPTER 1. General Introduction 2 PART I: ASTERACEAE CHAPTER 2. Multiple evolutionary radiations and phenotypic convergence in polyphyletic Indian Ocean Daisy Trees (Psiadia, Asteraceae) (in preparation for BMC Evolutionary Biology) 14 CHAPTER 3. Taxonomic rearrangements within Indian Ocean Daisy Trees (Psiadia, Asteraceae) and the resurrection of Frappieria (in preparation for Taxon) 34 PART II: MYRSINACEAE CHAPTER 4. Phylogenetics of the Mascarene endemic genus Badula relative to its Madagascan ally Oncostemum (Myrsinaceae) (accepted in Botanical Journal of the Linnean Society) 43 CHAPTER 5. Timing and tempo of evolutionary diversification in Myrsinaceae: Badula and Oncostemum in the Indian Ocean Island Biodiversity Hotspot (in preparation for BMC Evolutionary Biology) 54 PART III: MONIMIACEAE CHAPTER 6. Biogeography of the Monimiaceae (Laurales): a role for East Gondwana and long distance dispersal, but not West Gondwana (accepted in Journal of Biogeography) 72 CHAPTER 7 General Discussion 86 REFERENCES 91 i Contents. -

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -

Newsletter No
Newsletter No. 165 December 2015 Price: $5.00 AUSTRALASIAN SYSTEMATIC BOTANY SOCIETY INCORPORATED Council President Vice President Darren Crayn Daniel Murphy Australian Tropical Herbarium (ATH) Royal Botanic Gardens Victoria James Cook University, Cairns Campus Birdwood Avenue PO Box 6811, Cairns Qld 4870 Melbourne, Vic. 3004 Australia Australia Tel: (+61)/(0)7 4232 1859 Tel: (+61)/(0) 3 9252 2377 Email: [email protected] Email: [email protected] Secretary Treasurer Leon Perrie John Clarkson Museum of New Zealand Te Papa Tongarewa Queensland Parks and Wildlife Service PO Box 467, Wellington 6011 PO Box 975, Atherton Qld 4883 New Zealand Australia Tel: (+64)/(0) 4 381 7261 Tel: (+61)/(0) 7 4091 8170 Email: [email protected] Mobile: (+61)/(0) 437 732 487 Councillor Email: [email protected] Jennifer Tate Councillor Institute of Fundamental Sciences Mike Bayly Massey University School of Botany Private Bag 11222, Palmerston North 4442 University of Melbourne, Vic. 3010 New Zealand Australia Tel: (+64)/(0) 6 356 9099 ext. 84718 Tel: (+61)/(0) 3 8344 5055 Email: [email protected] Email: [email protected] Other constitutional bodies Hansjörg Eichler Research Committee Affiliate Society David Glenny Papua New Guinea Botanical Society Greg Leach Sarah Matthews Advisory Standing Committees [Vacancies to be filled by Council shortly] Financial Chair: Dan Murphy, Vice President Patrick Brownsey Grant application closing dates David Cantrill Hansjörg Eichler Research Fund: Bob Hill on March 14th and September 14th -

Tasmania - from the Wet West to the Dry East
This collection is maintained with the assistance of the Tasmania - from the wet west to the dry east. Regional Branch of the Australian Plant Society. Influences on the development of the Tasmanian plant mix Montane moorland and cool oceanic heathland When Gondwana existed as a super Geology of Tasmania Vegetation Map of Tasmania The Tasmanian highland vegetation developed in isolation from the Australian Alps. Even during ice continent, Australia and Tasmania, Africa, ages, hundreds of kilometres of lowland vegetation separated the two high altitude environments. South America, New Zealand and Antarctica shared many plant families and some Montane plants have to cope with wide temperature fluctuations, with periods of below 0°C and Genera. exposure to winds. Cold may be prolonged if the ground freezes. Plants may be blanketed by snow or BASS STRAIT the mountains by cloud. Snowmelt or clear weather can cause intense rays of light, resulting in high For example, the protea family has members temperature. Wind or sun can dry the plant and soil. in all those land masses except Antarctica. The Southern Africa panel covers the protea family more fully. Plants require moisture and warmth. Small hard leaves offer Tasmania was the last land mass to break protection from the drying away from Antarctica. The opening of the effects of sun and wind. Low gap between these land masses allowed the ocean to circulate growth avoids wind. Branches around Antarctica, cooling the earth’s climate and so locking up grow close together to shelter vast quantities of water as ice. the parts of each plant. -

World Heritage Values and to Identify New Values
FLORISTIC VALUES OF THE TASMANIAN WILDERNESS WORLD HERITAGE AREA J. Balmer, J. Whinam, J. Kelman, J.B. Kirkpatrick & E. Lazarus Nature Conservation Branch Report October 2004 This report was prepared under the direction of the Department of Primary Industries, Water and Environment (World Heritage Area Vegetation Program). Commonwealth Government funds were contributed to the project through the World Heritage Area program. The views and opinions expressed in this report are those of the authors and do not necessarily reflect those of the Department of Primary Industries, Water and Environment or those of the Department of the Environment and Heritage. ISSN 1441–0680 Copyright 2003 Crown in right of State of Tasmania Apart from fair dealing for the purposes of private study, research, criticism or review, as permitted under the Copyright Act, no part may be reproduced by any means without permission from the Department of Primary Industries, Water and Environment. Published by Nature Conservation Branch Department of Primary Industries, Water and Environment GPO Box 44 Hobart Tasmania, 7001 Front Cover Photograph: Alpine bolster heath (1050 metres) at Mt Anne. Stunted Nothofagus cunninghamii is shrouded in mist with Richea pandanifolia scattered throughout and Astelia alpina in the foreground. Photograph taken by Grant Dixon Back Cover Photograph: Nothofagus gunnii leaf with fossil imprint in deposits dating from 35-40 million years ago: Photograph taken by Greg Jordan Cite as: Balmer J., Whinam J., Kelman J., Kirkpatrick J.B. & Lazarus E. (2004) A review of the floristic values of the Tasmanian Wilderness World Heritage Area. Nature Conservation Report 2004/3. Department of Primary Industries Water and Environment, Tasmania, Australia T ABLE OF C ONTENTS ACKNOWLEDGMENTS .................................................................................................................................................................................1 1. -

Invasion and Management of a Woody Plant, Lantana Camara L., Alters Vegetation Diversity Within Wet Sclerophyll Forest in Southeastern Australia
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Ben Gooden University of Wollongong, [email protected] Kris French University of Wollongong, [email protected] Peter J. Turner Department of Environment and Climate Change, NSW Follow this and additional works at: https://ro.uow.edu.au/scipapers Part of the Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Social and Behavioral Sciences Commons Recommended Citation Gooden, Ben; French, Kris; and Turner, Peter J.: Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia 2009. https://ro.uow.edu.au/scipapers/4953 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Abstract Plant invasions of natural communities are commonly associated with reduced species diversity and altered ecosystem structure and function. This study investigated the effects of invasion and management of the woody shrub Lantana camara (lantana) in wet sclerophyll forest on the south-east coast of Australia. The effects of L. camara invasion and management on resident vegetation diversity and recruitment were determined as well as if invader management initiated community recovery. Vascular plant species richness, abundance and composition were surveyed and compared across L. -

(OUV) of the Wet Tropics of Queensland World Heritage Area
Handout 2 Natural Heritage Criteria and the Attributes of Outstanding Universal Value (OUV) of the Wet Tropics of Queensland World Heritage Area The notes that follow were derived by deconstructing the original 1988 nomination document to identify the specific themes and attributes which have been recognised as contributing to the Outstanding Universal Value of the Wet Tropics. The notes also provide brief statements of justification for the specific examples provided in the nomination documentation. Steve Goosem, December 2012 Natural Heritage Criteria: (1) Outstanding examples representing the major stages in the earth’s evolutionary history Values: refers to the surviving taxa that are representative of eight ‘stages’ in the evolutionary history of the earth. Relict species and lineages are the elements of this World Heritage value. Attribute of OUV (a) The Age of the Pteridophytes Significance One of the most significant evolutionary events on this planet was the adaptation in the Palaeozoic Era of plants to life on the land. The earliest known (plant) forms were from the Silurian Period more than 400 million years ago. These were spore-producing plants which reached their greatest development 100 million years later during the Carboniferous Period. This stage of the earth’s evolutionary history, involving the proliferation of club mosses (lycopods) and ferns is commonly described as the Age of the Pteridophytes. The range of primitive relict genera representative of the major and most ancient evolutionary groups of pteridophytes occurring in the Wet Tropics is equalled only in the more extensive New Guinea rainforests that were once continuous with those of the listed area.