Gemmology2013 / Volume 33 / Nos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
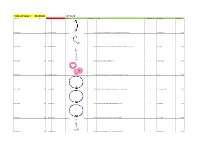
Total Lot Value = $8,189.80 LOT #128 Location Id Lot # Item Id Sku Image Store Price Model Store Quantity Classification Total Value
Total Lot Value = $8,189.80 LOT #128 location_id Lot # item_id sku Image store_price model store_quantity classification Total Value A07-S05-D001 128 182363 TICB-1001 $13.95 16G 5/16" Internally Threaded Implant Grade Titanium Curved Barbell 8 Curved Barbell $111.60 A07-S05-D003 128 186942 NR-1316 $8.95 Purple 2mm Bezel-Set Synthetic Opal Rose Gold-Tone Steel Nose Ring Twist 1 Nose Ring $8.95 A07-S05-D005 128 65492 A208 $11.99 Celtic Swirl Reverse Belly Button Ring 4 Belly Ring Sale $47.96 A07-S05-D006 128 104247 PLG-1345-14 $10.95 9/16" Pink Heart-Shaped Cut Out Flexible Silicone Tunnel Plugs Pair 4 Plugs $43.80 A07-S05-D007 128 65494 D103 $5.95 16G 3/8 - Anodized Rainbow Titanium-Plated Captive Bead Ring 3 Captive Ring , Daith $17.85 A07-S05-D008 128 65496 D103b $5.95 Rainbow Titanium-Plated Captive Bead Ring - 14G 1/2 1 Captive Ring $5.95 A07-S05-D010 128 65499 D103d $4.99 16G 5/8 - Rainbow Anodized Steel Captive Bead Ring 51 Captive Ring $254.49 A07-S05-D011 128 87697 BR-1429 $11.99 Pink CZ Crown & Flower Dangle Belly Button Navel Ring 2 Belly Ring Sale $23.98 A07-S05-D012 128 64066 BR-1496 $11.99 Purple CZ Crown & Flower Dangle Belly Button Navel Ring 4 Belly Ring Sale $47.96 A07-S05-D013 128 65502 D111 $5.99 Spiky -Ball Twister - Body Jewelry 14 Gauge 43 Twister Barbell Sale $257.57 A07-S05-D014 128 104271 PLG-1345-16 $10.95 5/8" - Pink Heart-Shaped Cut Out Flexible Silicone Tunnel Plugs - Pair 2 Plugs $21.90 A07-S05-D015 128 104185 PLG-1345-4 $8.95 6G - Pink Heart-Shaped Cut Out Flexible Silicone Tunnel Plugs - Pair 2 Plugs $17.90 -

Kiss the Ring New York
Kiss the ring new york Fine Jewelry from the New York Diamond District. Ebony & Gold Link Bracelet $3, Gold Link Necklace from $3, Pearl Kiss Necklace $3, Kiss the Ring New York. likes · 11 talking about this. Custom Fine Jewelry from the New York Diamond District Kiss the Ring New York. likes · 1 talking about this. Custom Fine Jewelry from the New York Diamond District Fine jewelry expert and Founder of Kiss The Ring New York, Hattie Gruber is the woman behind Manhattan's biggest engagement ring and fine. Kiss the Ring NY on 15 W 47th St, New York, NY. Lunch, dinner, groceries, office supplies or anything else. Our Postmates deliver from all your favorites places. Gabriella studied sculpture at New York's Pratt Institute, learning to make wax models for casting, Oval White Rose Cut Diamond Ring,Gabriella Kiss. NEW YORK CITY: Kiss The Ring – Labor Day Weekend Day Party. August 26, by Genese Jamilah · Facebook. Free Shipping and Free Returns on Eva Fehren Kissing Claw Ring at Exclusively Ours!Handcrafted in New York City, Eva Fehren's pavé. Some people have a hard time taking compliments. I am not one of those people. So when I read Jessica Mischner's post about Kiss the Ring. I was introduced to the Gossip Girl series several years ago when I read the books by Cecily von Ziegesar. I told my mother about them and she. Shop our selection of engagement rings, wedding rings, diamonds, gemstones, metals, watches and more. Kay Jewelers carries a wide selection of looks, from. Marilyn Manson Injured Onstage in New York, Taken Away on Stretcher "Kiss the Ring" is a messy project connected only by DJ Khaled's. -
Phenomenal Gemstones Possess Striking Optical Effects, Making Them Truly a Sight for Sore Eyes
THE PHENOMENAL PROPERTIES OF GEMS Phenomenal gemstones possess striking optical effects, making them truly a sight for sore eyes. Here is GIA’s guide to understanding what makes each phenomenon so uniquely brilliant. ASTERISM CROSSING BANDS OF REFLECTED LIGHT CREATE A SIX-RAYED STAR-LIKE APPEARANCE. ASTERISM OCCURS IN THE DOME OF A CABOCHON, AND CAN BE SEEN IN GEMS LIKE RUBIES AND SAPPHIRES. ADULARESCENCE THE SAME SCATTERING OF LIGHT THAT MAKES THE SKY BLUE CREATES A MILKY, BLUISH-WHITE GLOW, LIKE MOONLIGHT SHINING THROUGH A VEIL OF CLOUDS. MOONSTONE IS THE ONLY GEM THAT DISPLAYS IT. AVENTURESCENCE FOUND IN NATURAL GEMS LIKE SUNSTONE FELDSPAR AND AVENTURINE QUARTZ, IT DISPLAYS A GLITTERY EFFECT CAUSED BY LIGHT REFLECTING FROM SMALL, FLAT INCLUSIONS. CHATOYANCY OTHERWISE KNOWN AS THE “CAT’S EYE” EFFECT, BANDS OF LIGHT ARE CAUSED BY THE REFLECTION OF LIGHT FROM MANY PARALLEL, NEEDLE-LIKE INCLUSIONS INSIDE A CABOCHON. NOTABLE GEMS THAT DISPLAY CHATOYANCY INCLUDE CAT’S EYE TOURMALINE AND CAT’S EYE CHRYSOBERYL. IRIDESCENCE ALSO SEEN IN SOAP BUBBLES AND OIL SLICKS, IT’S A RAINBOW EFFECT THAT IS CREATED WHEN LIGHT IS BROKEN UP INTO DIFFERENT COLORS. LOOK FOR IT IN FIRE AGATE AND OPAL AMMONITE (KNOWN BY THE TRADE AS AMMOLITE). LABR ADORESCENCE A BROAD FLASH OF COLOR THAT APPEARS IN LABRADORITE FELDSPAR, IT’S CAUSED BY LIGHT INTERACTING WITH THIN LAYERS IN THE STONE, AND DISAPPEARS WHEN THE GEM IS MOVED. INSIDER’S TIP: THE MOST COMMON PHENOMENAL COLOR IN LABRADORITE IS BLUE. PLAY OF COLOR THE FLASHING RAINBOW-LIKE COLORS IN OPAL THAT FLASH AT YOU AS YOU TURN THE STONE OR MOVE AROUND IT. -

Distinctive Designs
Distinctive Designs Brides Ring in an Era of Self-Expression by Stacey Marcus Diamond rings have been sporting the hands of newly can elevate a traditional setting to an entirely new level. While engaged women since ancient times. In the mid 1940s, diamonds are always in vogue for engagement rings, millen- DeBeers revived the ritual with its famous “Diamonds Are nials are also opting for nontraditional stones, such as color- Forever” campaign. Today’s brides are blazing new trails by changing alexandrite, beautiful tanzanite, black opal, and even selecting nontraditional wedding rings that express their aquamarine. We are in an age when anything goes, and brides individual style. are embracing the idea of individuality and self-expression!” True Colors says Jordan Fine, CEO of JFINE. “Brides today want to be unique and they don’t mind taking Kathryn Money, vice president of strategy and merchandising chances with colors, settings, and stones. When it comes to at Brilliant Earth agrees: “Customers are seeking products that choosing an engagement ring, natural pink, blue, and even express their individuality and are increasingly drawn towards green diamonds are trending. These precious and rare dia- uniqueness. They want a ring that isn’t like everyone else’s, monds originate from only a few mines in the world, and they which we’re seeing manifested in many different ways, such 34 Spring 2018 BRIDE&GROOM as choosing a distinctive ring setting, using colored gemstones in lieu of a diamond, using a fancy (non-round) diamond, or opting for rose gold.” Money adds that 16% of respondents in a recent survey they conducted favored a colored gemstone engagement ring over a diamond. -

Gemstones in Metal Clay
Gemstones in Metal Clay Many natural gemstones can be set into metal clay and fired in place. Other gemstones will not survive the heat of a kiln and should be set after firing. These charts show the results of kiln and torch tests that have been performed on both natural and synthetic gemstones, adapted with permission from the original testing by Kevin Whitmore of Rio Grande. This information is for reference and should be used as a guide. There is always some risk of losing a natural gemstone even if others of it’s kind have survived in the past. Gemstones may have internal flaws that can be liquid or gaseous filled, or contain crystals of other materials that can cause the gemstone to fail where it usually does not. This guide aims to help metal clay artists sort out gemstones that are known to survive under fire from those that are not. Gemstones are minerals that are classified into groups based upon the constancy of their major properties. Each mineral family has one or more varieties contained within the group. When we sort the tested gemstones according to their mineral group, it becomes clear that an easy way to gauge the survivability of a gemstone is to look at the results of other varieties within that same group. Aquamarine and emerald, for example, are both varieties of the beryl group of minerals. The result of tests done on aquamarine and emerald indicate that minerals in the beryl group will not survive kiln heating. There are exceptions, as there always are in the natural world, but in general this method can be reliable for many varieties. -

Archaeological Journal on Episcopal Rings
This article was downloaded by: [Northwestern University] On: 14 February 2015, At: 08:09 Publisher: Routledge Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Archaeological Journal Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/raij20 On Episcopal Rings Edmund Waterton K.Ch., F S.A., F.S.A.S., M.R.I.A. Published online: 10 Jul 2014. To cite this article: Edmund Waterton K.Ch., F S.A., F.S.A.S., M.R.I.A. (1863) On Episcopal Rings, Archaeological Journal, 20:1, 224-238, DOI: 10.1080/00665983.1863.10851251 To link to this article: http:// dx.doi.org/10.1080/00665983.1863.10851251 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content. -

MAGAZINE • LEAWOOD, KS SPECIAL EDITION 2016 ISSUE 3 Fine Jewelers Magazine
A TUFTS COMMUNICATIONS fine jewelry PUBLICATION MAZZARESE MAGAZINE • LEAWOOD, KS SPECIAL EDITION 2016 ISSUE 3 fine jewelers magazine Chopard: Racing Special The New Ferrari 488 GTB The Natural Flair of John Hardy Black Beauties Omega’s New 007 SPECIAL EDITION 2016 • ISSUE 3 MAZZARESE FINE JEWELERS MAGAZINE • SPECIAL EDITION 2016 welcome It is our belief that we have an indelible link with the past and a responsibility to the future. In representing the fourth generation of master jewelers and craftsmen, and even as the keepers of a second generation family business, we believe that the responsibility to continually evolve and develop lies with us. We endeavor to always stay ahead of the latest jewelry and watch trends and innovations. We stay true to our high standards and objectives set forth by those that came before us by delivering a jewelry experience like no other; through the utmost attention to service, knowledge and value. Every great story begins with a spark of inspiration. We are reminded day after day of our spark of inspiration: you, our esteemed customer. Your stories help drive our passion to pursue the finest quality pieces. It is a privilege that we are trusted to provide the perfect gift; one that stands the test of time and is passed along through generations. We find great joy in assisting the eager couple searching for the perfect engagement ring, as they embark on a lifetime of love, helping to select a quality timepiece, or sourcing a rare jewel to mark and celebrate a milestone. We are dedicated to creating an experience that nurtures relationships and allows those who visit our store to enter as customers, but leave as members of our family. -

The Journal of Gemmology Editor: Dr R.R
he Journa TGemmolog Volume 25 No. 8 October 1997 The Gemmological Association and Gem Testing Laboratory of Great Britain Gemmological Association and Gem Testing Laboratory of Great Britain 27 Greville Street, London Eel N SSU Tel: 0171 404 1134 Fax: 0171 404 8843 e-mail: [email protected] Website: www.gagtl.ac.uklgagtl President: Professor R.A. Howie Vice-Presidents: LM. Bruton, Af'. ram, D.C. Kent, R.K. Mitchell Honorary Fellows: R.A. Howie, R.T. Liddicoat Inr, K. Nassau Honorary Life Members: D.). Callaghan, LA. lobbins, H. Tillander Council of Management: C.R. Cavey, T.]. Davidson, N.W. Decks, R.R. Harding, I. Thomson, V.P. Watson Members' Council: Aj. Allnutt, P. Dwyer-Hickey, R. fuller, l. Greatwood. B. jackson, J. Kessler, j. Monnickendam, L. Music, l.B. Nelson, P.G. Read, R. Shepherd, C.H. VVinter Branch Chairmen: Midlands - C.M. Green, North West - I. Knight, Scottish - B. jackson Examiners: A.j. Allnutt, M.Sc., Ph.D., leA, S.M. Anderson, B.Se. (Hons), I-CA, L. Bartlett, 13.Se, .'vI.phil., I-G/\' DCi\, E.M. Bruton, FGA, DC/\, c.~. Cavey, FGA, S. Coelho, B.Se, I-G,\' DGt\, Prof. A.T. Collins, B.Sc, Ph.D, A.G. Good, FGA, f1GA, Cj.E. Halt B.Sc. (Hons), FGr\, G.M. Howe, FG,'\, oo-, G.H. jones, B.Se, PhD., FCA, M. Newton, B.Se, D.PhiL, H.L. Plumb, B.Sc., ICA, DCA, R.D. Ross, B.5e, I-GA, DGA, P..A.. Sadler, 13.5c., IGA, DCA, E. Stern, I'GA, DC/\, Prof. I. -

June 2008 'Tairus' Created Gems: “Part I - Beryl”
Sponsored by Ministry of Commerce & Industry Volume 51 Lab Information Circular June 2008 'Tairus' Created Gems: “Part I - Beryl” In the Volume 49 of Lab Information Circular, November 2007, PleochroismPleochroism: : Dichroism varied from weak in aquamarine to we reported “Synthetic Beryl of Paraiba colour” produced by very strong in darker coloured varieties like purple pink or Tairus Co. Ltd. in order to take the advantage of the popularity of orange red (see figure 2). 'Paraiba' tourmalines. Thereafter, we procured sets of various coloured beryls and corundum from the same manufacturer for our research and reference purpose. The study of these sets has been divided into two parts; this issue describes the study of beryl set, while the study of corundum set will be published in the next issue. 2.a 2.c 2.e The Beryl set consisted of five colour varieties (figure 1), namely, green (emerald), light blue (aquamarine), electric greenish blue (paraiba type), purple pink and orange red. All specimens were facetted as square step and measured 6 X 6 mm. 2.b 2.d 2.f Figure 2 Pleochroic colours of Tairus created Beryls Absorption Spectrum:Spectrum : The spectrum varied as per the colour variety; emerald showed a typical chromium spectrum while 'paraiba' colour displayed a strong band at around 430 nm. Purple pink and orange red varieties exhibited strong bands at 460 to 490 and 550 to 600 nm. UV fluorescence:fluorescence : All samples were inert to long wave as well as short wave UV light. Figure 1 Chelsea Filter Reactioneaction: : Emerald revealed a red glow while all Standard gemmological tests and advanced analysis on FTIR other varieties were either inert or did not displayed any reaction. -

Murostar-Katalog-2018-2019.Pdf
Material B2B Großhandel für Piercing- und Tattoo- B2B Wholesale for body piercing and Unsere Qualität ist Ihre Zufriedenheit Our quality is your satisfaction About Us studios, sowie Schmuck- und Juwelier- tattoo studios as well as jewelry stores vertriebe Oberste Priorität hat bei uns die reibungslose Abwicklung Our top priority is to provide a smooth transaction and fast Titan Produkte entsprechen dem Grad 23 (Ti6AL 4V Eli) und Titanium products correspond to titanium grade 23 (Ti6Al und schnelle Lieferung Ihrer Ware. Daher verlassen ca. 95 % delivery. Therefore, approximately 95% of all orders are sind generell hochglanzpoliert. Sterilisierbar. 4V Eli) and are generally high polished. For sterilization. aller Aufträge unser Lager noch am selben Tag. shipped out on the same day. Black Titan besteht grundsätzlich aus Titan Grad 23 (Ti6AL Black Titanium is composed of titanium grade 23 (Ti6Al 4V Unser dynamisches, kundenorientiertes Team sowie ausge- Our dynamic, customer-oriented team and excellent expe- 4V Eli) und ist zusätzlich mit einer PVD Titanium Beschich- Eli) and is additionally equipped with a PVD Black Titanium zeichnete Erfahrungswerte garantieren Ihnen einen hervor- rience guarantee excellent service and expert advice. tung geschwärzt. Sterilisierbar. Coating. For sterilization. ragenden Service und kompetente Beratung. Steel Schmuck besteht grundsätzlich aus Chirurgenstahl Steel jewelry is composed of 316L Surgical Steel and is also Sie bestellen schnell und unkompliziert online, telefo- Your order can be placed quickly and easily online, by 316L und ist ebenfalls hochglanzpoliert. Sterilisierbar. high polished. For sterilization. nisch, per Fax oder Email mittels unseres einfachen Ex- phone, fax or email using our simple Express Number press-Nummern-Systems ohne Angabe von Farb- oder Grö- System only without providing colors or sizes. -

SYNTHETIC GEM MATERIALS in the 2000S GEMS & GEMOLOGY WINTER 2010 Figure 1
SYNTHETIC GEM MATERIALS INTHE2000S: A DECADE IN REVIEW Nathan Renfro, John I. Koivula, Wuyi Wang, and Gary Roskin The first decade of the 2000s brought a constant flow of previously known synthetics into the marketplace, but little in the way of new technology. The biggest development was the commer- cial introduction of faceted single-crystal gem-quality CVD synthetic diamonds. A few other inter- esting and noteworthy synthetics, such as Malossi hydrothermal synthetic emeralds and Mexifire synthetic opals, also entered the market. Identification of synthetic gem materials continued to be an important function of—and, in some cases, challenge for—gemologists worldwide. he development of synthetics and the method- lished literature that the most significant develop- ologies used to detect new and existing materi- ments—and the focus of most research—during this Tals is of great importance to the international decade involved the production of gem-quality syn- gem community. Indeed, whether a synthetic gem thetic diamonds, primarily those grown by the com- was grown in the 2000s or the 1880s, today’s gemol- paratively new CVD (chemical vapor deposition) ogists must still be prepared to deal with it. Many process. Who can forget the September 2003 cover of synthetic gems were prominent in the marketplace Wired magazine, with a diamond-pavéd “supermod- in the first decade of the 2000s (see, e.g., figure 1). el” next to the headlines “$5 a carat. Flawless. Made The decade also saw some new synthetics. Among in a lab.”? This article proclaimed that “The dia- the synthetic colored stones introduced was the mond wars have begun,” and touted the potential for Malossi hydrothermal synthetic emerald (Adamo et outright cheap but extremely high-quality colorless al., 2005), which was gemologically similar to both and fancy-colored synthetic diamonds grown by two Russian synthetic emeralds and those manufactured very different processes (CVD and HPHT). -

Reflective Index Reference Chart
REFLECTIVE INDEX REFERENCE CHART FOR PRESIDIUM DUO TESTER (PDT) Reflective Index Refractive Reflective Index Refractive Reflective Index Refractive Gemstone on PDT/PRM Index Gemstone on PDT/PRM Index Gemstone on PDT/PRM Index Fluorite 16 - 18 1.434 - 1.434 Emerald 26 - 29 1.580 - 1.580 Corundum 34 - 43 1.762 - 1.770 Opal 17 - 19 1.450 - 1.450 Verdite 26 - 29 1.580 - 1.580 Idocrase 35 - 39 1.713 - 1.718 ? Glass 17 - 54 1.440 - 1.900 Brazilianite 27 - 32 1.602 - 1.621 Spinel 36 - 39 1.718 - 1.718 How does your Presidium tester Plastic 18 - 38 1.460 - 1.700 Rhodochrosite 27 - 48 1.597 - 1.817 TL Grossularite Garnet 36 - 40 1.720 - 1.720 Sodalite 19 - 21 1.483 - 1.483 Actinolite 28 - 33 1.614 - 1.642 Kyanite 36 - 41 1.716 - 1.731 work to get R.I. values? Lapis-lazuli 20 - 23 1.500 - 1.500 Nephrite 28 - 33 1.606 - 1.632 Rhodonite 37 - 41 1.730 - 1.740 Reflective indices developed by Presidium can Moldavite 20 - 23 1.500 - 1.500 Turquoise 28 - 34 1.610 - 1.650 TP Grossularite Garnet (Hessonite) 37 - 41 1.740 - 1.740 be matched in this table to the corresponding Obsidian 20 - 23 1.500 - 1.500 Topaz (Blue, White) 29 - 32 1.619 - 1.627 Chrysoberyl (Alexandrite) 38 - 42 1.746 - 1.755 common Refractive Index values to get the Calcite 20 - 35 1.486 - 1.658 Danburite 29 - 33 1.630 - 1.636 Pyrope Garnet 38 - 42 1.746 - 1.746 R.I value of the gemstone.