Curiosity Guide #308 Candy Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adult Contemporary Radio at the End of the Twentieth Century
University of Kentucky UKnowledge Theses and Dissertations--Music Music 2019 Gender, Politics, Market Segmentation, and Taste: Adult Contemporary Radio at the End of the Twentieth Century Saesha Senger University of Kentucky, [email protected] Digital Object Identifier: https://doi.org/10.13023/etd.2020.011 Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Senger, Saesha, "Gender, Politics, Market Segmentation, and Taste: Adult Contemporary Radio at the End of the Twentieth Century" (2019). Theses and Dissertations--Music. 150. https://uknowledge.uky.edu/music_etds/150 This Doctoral Dissertation is brought to you for free and open access by the Music at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Music by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Final Version
This research has been supported as part of the Popular Music Heritage, Cultural Memory and Cultural Identity (POPID) project by the HERA Joint Research Program (www.heranet.info) which is co-funded by AHRC, AKA, DASTI, ETF, FNR, FWF, HAZU, IRCHSS, MHEST, NWO, RANNIS, RCN, VR and The European Community FP7 2007–2013, under ‘the Socio-economic Sciences and Humanities program’. ISBN: 978-90-76665-26-9 Publisher: ERMeCC, Erasmus Research Center for Media, Communication and Culture Printing: Ipskamp Drukkers Cover design: Martijn Koster © 2014 Arno van der Hoeven Popular Music Memories Places and Practices of Popular Music Heritage, Memory and Cultural Identity *** Popmuziekherinneringen Plaatsen en praktijken van popmuziekerfgoed, cultureel geheugen en identiteit Thesis to obtain the degree of Doctor from the Erasmus University Rotterdam by command of the rector magnificus Prof.dr. H.A.P Pols and in accordance with the decision of the Doctorate Board The public defense shall be held on Thursday 27 November 2014 at 15.30 hours by Arno Johan Christiaan van der Hoeven born in Ede Doctoral Committee: Promotor: Prof.dr. M.S.S.E. Janssen Other members: Prof.dr. J.F.T.M. van Dijck Prof.dr. S.L. Reijnders Dr. H.J.C.J. Hitters Contents Acknowledgements 1 1. Introduction 3 2. Studying popular music memories 7 2.1 Popular music and identity 7 2.2 Popular music, cultural memory and cultural heritage 11 2.3 The places of popular music and heritage 18 2.4 Research questions, methodological considerations and structure of the dissertation 20 3. The popular music heritage of the Dutch pirates 27 3.1 Introduction 27 3.2 The emergence of pirate radio in the Netherlands 28 3.3 Theory: the narrative constitution of musicalized identities 29 3.4 Background to the study 30 3.5 The dominant narrative of the pirates: playing disregarded genres 31 3.6 Place and identity 35 3.7 The personal and cultural meanings of illegal radio 37 3.8 Memory practices: sharing stories 39 3.9 Conclusions and discussion 42 4. -

MTO 11.4: Spicer, Review of the Beatles As Musicians
Volume 11, Number 4, October 2005 Copyright © 2005 Society for Music Theory Mark Spicer Received October 2005 [1] As I thought about how best to begin this review, an article by David Fricke in the latest issue of Rolling Stone caught my attention.(1) Entitled “Beatles Maniacs,” the article tells the tale of the Fab Faux, a New York-based Beatles tribute group— founded in 1998 by Will Lee (longtime bassist for Paul Schaffer’s CBS Orchestra on the Late Show With David Letterman)—that has quickly risen to become “the most-accomplished band in the Beatles-cover business.” By painstakingly learning their respective parts note-by-note from the original studio recordings, the Fab Faux to date have mastered and performed live “160 of the 211 songs in the official canon.”(2) Lee likens his group’s approach to performing the Beatles to “the way classical musicians start a chamber orchestra to play Mozart . as perfectly as we can.” As the Faux’s drummer Rich Pagano puts it, “[t]his is the greatest music ever written, and we’re such freaks for it.” [2] It’s been over thirty-five years since the real Fab Four called it quits, and the group is now down to two surviving members, yet somehow the Beatles remain as popular as ever. Hardly a month goes by, it seems, without something new and Beatle-related appearing in the mass media to remind us of just how important this group has been, and continues to be, in shaping our postmodern world. For example, as I write this, the current issue of TV Guide (August 14–20, 2005) is a “special tribute” issue commemorating the fortieth anniversary of the Beatles’ sold-out performance at New York’s Shea Stadium on August 15, 1965—a concert which, as the magazine notes, marked the “dawning of a new era for rock music” where “[v]ast outdoor shows would become the superstar standard.”(3) The cover of my copy—one of four covers for this week’s issue, each featuring a different Beatle—boasts a photograph of Paul McCartney onstage at the Shea concert, his famous Höfner “violin” bass gripped in one hand as he waves to the crowd with the other. -

Pop/Rock/Dance Pop/Rock/Dance
pop/rock/dance A Little Less Conversation (Elvis) All About That Bass (Meghan Trainor) All Day All Night (Kinks) All My Loving (Beatles) All Shook Up (Elvis) All The Small Things (Blink 182) Angels (Robbie Wiliams) Are You Gonna Be My Girl (Jet) Are You Gonna Go My Way Lenny Kravitz) Beautiful Day (U2) Bi llie Jean (Michael Jackson) Blister In The Sun (Violent Femmes) Blurred Lines (Robin Thicke) Boom! Shake The Room. (Jazzy Jeff & The Fresh Prince) Brown Eyed Girl (Van Morrison) Brown Sugar (Rolling Stones) Bust A Move (Young MC) Call Me Maybe (Carly Rae J epson) Can’t Get Enough Of Your Love Baby (Barry White) Can’t Buy Me Love (Beatles) Can’t Take My Eyes Off You (Frankie Vallli) Celebration (Kool and the Gang) Chasing Cars (Snow Patrol) Crazy Little Thing Called Love (Queen) Dancing Queen (Abba) December ’63 (Frankie Valli) Dock Of The Bay (Otis Redding) Eagle Rock (Daddy Cool) Eight Days A Week (Beatles) Everybody Needs Somebody (Wilson Picket) Every Little Thing She Does Is Magic (Police) Faith (George Michael) Feel So Close (Calvin Harris) Fight For Your Right To Party (Beastie Boys) Fireworks Katy Perry) Friday I’m In Love (Cure) Funky Cold Medina (Tone Loc) Get Lucky (Daft Punk) Get This Party Started (Pink) Gimme Some Lovin’ (Spencer Davis Group) Good Feeling (Flo Rida) Good Riddance (Time Of Your Life) Great Balls Of Fire (Jerry Lee Lewis) Groove Is In The Heart (Deelite) Happy (Pharrell Williams) Hard To Handle (Otis Redding) Hawaii Five-O (The Ventures) Heroes (David Bowie) Hey Soul Sister (Train) Hold On I’m Coming (Sam -

History 371 Society, Culture, and Rock and Roll Spring 2011 Phone: 463
History 371 Society, Culture, and Rock and Roll Spring 2011 Professor Michael A. Morrison Office Hours: Monday: University Hall 123 12:00-1:00 other days/times by appointment Email: [email protected] (work) [email protected] (home) Phone: 463-0087 (home) Teaching Assistants: David Weir Mauricio Castro REC 421 REC 402 [email protected] [email protected] Office Hour: Monday 2:00-3:00 Office Hour: Tuesday 2:00-3:00 & by appointment & by appointment STUDENT’S LAST NAME: A-K STUDENT’S LAST NAME: L-Z This class will survey the social and cultural fabric of post-World War II United States through the prism of music—rock and roll music. At one level the class will survey trends and styles in rock, focusing first on the artists and groups who gave rise to this hybrid form of music from its country and blues roots. It will then track the rise of rock and roll in the 1950s and the corporate, political, and social backlash against it. The focus on the 1960s will be on music as an expression and extension of the social, cultural, and political changes of that decade. Finally, the class will examine the paradoxical developments of the evolution of music videos (read: MTV) with the emergence of an abrasive, often angry music (read: punk/grunge/rap) by the end of the 1970s and into the 1990s. In the end, this class will examine and explain the technological, business, and social forces that helped cement rock’s position in Western popular culture. At another, deeper level, by placing this tradition of popular music in its historic context, the class will look at the problematic and interrelated issues of music, business, politics, gender, race, class, and culture in the postwar era. -

The Music (And More) 2019 Quarter 3 Report
The Music (and More) 2019 Quarter 3 Report Report covers the time period of July 1st to Kieran Robbins & Chief - "Sway" [glam rock] September 30th, 2019. I inadvertently missed Troy a few before that time period, which were brought to my attention by fans, bands & Moriah Formica - "I Don't Care What You others. The missing are listed at the end, along with an Think" (single) [hard rock] Albany End Note… Nine Votes Short - "NVS: 09/03/2019" [punk rock] RECORDINGS: Albany Hard Rock / Metal / Punk Psychomanteum - "Mortal Extremis" (single track) Attica - "Resurected" (EP) [hardcore metal] Albany [thrash prog metal industrial] Albany Between Now and Forever - "Happy" (single - Silversyde - "In The Dark" [christian gospel hard rock] Mudvayne cover) [melodic metal rock] Albany Troy/Toledo OH Black Electric - "Black Electric" [heavy stoner blues rock] Scotchka - "Back on the Liquor" | "Save" (single tracks) Voorheesville [emo pop punk] Albany Blood Blood Blood - "Stranglers” (single) [darkwave Somewhere To Call Home - "Somewhere To Call Home" horror synthpunk] Troy [nu-metalcore] Albany Broken Field Runner – "Lay My Head Down" [emo pop Untaymed - "Lady" (single track) [british hard rock] punk] Albany / LA Colonie Brookline - "Live From the Bunker - Acoustic" (EP) We’re History - "We’re History" | "Pop Tarts" - [acoustic pop-punk alt rock] Greenwich "Avalanche" (singles) [punk rock pop] Saratoga Springs Candy Ambulance - "Traumantic" [alternative grunge Wet Specimens - "Haunted Flesh" (EP) [hardcore punk] rock] Saratoga Springs Albany Craig Relyea - "Between the Rain" (single track) Rock / Pop [modern post-rock] Schenectady Achille - "Superman (A Song for Mora)" (single) [alternative pop rock] Albany Dead-Lift - "Take It or Leave It" (single) [metal hard rock fusion] Schenectady Caramel Snow - "Wheels Are Meant To Roll Away" (single track) [shoegaze dreampop] Delmar Deep Slut - "u up?" (3-song) [punk slutcore rap] Albany Cassandra Kubinski - "DREAMS (feat. -

Glam Rock by Barney Hoskyns 1
Glam Rock By Barney Hoskyns There's a new sensation A fabulous creation, A danceable solution To teenage revolution Roxy Music, 1973 1: All the Young Dudes: Dawn of the Teenage Rampage Glamour – a word first used in the 18th Century as a Scottish term connoting "magic" or "enchantment" – has always been a part of pop music. With his mascara and gold suits, Elvis Presley was pure glam. So was Little Richard, with his pencil moustache and towering pompadour hairstyle. The Rolling Stones of the mid-to- late Sixties, swathed in scarves and furs, were unquestionably glam; the group even dressed in drag to push their 1966 single "Have You Seen Your Mother, Baby, Standing in the Shadow?" But it wasn't until 1971 that "glam" as a term became the buzzword for a new teenage subculture that was reacting to the messianic, we-can-change-the-world rhetoric of late Sixties rock. When T. Rex's Marc Bolan sprinkled glitter under his eyes for a TV taping of the group’s "Hot Love," it signaled a revolt into provocative style, an implicit rejection of the music to which stoned older siblings had swayed during the previous decade. "My brother’s back at home with his Beatles and his Stones," Mott the Hoople's Ian Hunter drawled on the anthemic David Bowie song "All the Young Dudes," "we never got it off on that revolution stuff..." As such, glam was a manifestation of pop's cyclical nature, its hedonism and surface show-business fizz offering a pointed contrast to the sometimes po-faced earnestness of the Woodstock era. -

Music in the UK (Worksheet)
ПОРТАЛ ДЛЯ ПРЕПОДАВАТЕЛЕЙ АНГЛИЙСКОГО HTTP://SKYTEACH.RU/ Music in the UK (worksheet) Activity 1 Look at the pictures. What do these pictures have in common? How often do you listen to music? Where? What types of music do you know? What kind of music do you like and don’t like? Why? Activity 2 Look at the pictures. Which singers and bands do you know? Which their songs have you heard? Spice Girls Queen The Rolling stones Adele Ed Sheeran Elton John Pink Floyd The Beatles Picture taken from https://pixabay.com and https://en.wikipedia.org Created and designed by Maria Tsedrik for Skyeng, 2018 © ПОРТАЛ ДЛЯ ПРЕПОДАВАТЕЛЕЙ АНГЛИЙСКОГО HTTP://SKYTEACH.RU/ Amy Winehouse Oasis One Direction Coldplay Complete the table with the name of the artists or bands. Dates Name Type of music 1962 - now 1965 - now 1960-1970 1970 - now 1965 - 1995 1991 - 2009 1994 - 2000 2003-2011 1998-now 2006 - now 2010-2016 2011 - now Watch the video and check. Picture taken from https://en.wikipedia.org Created and designed by Maria Tsedrik for Skyeng, 2018 © ПОРТАЛ ДЛЯ ПРЕПОДАВАТЕЛЕЙ АНГЛИЙСКОГО HTTP://SKYTEACH.RU/ Activity 3 Most famous British bands and musicians quiz. 1) What band is called “Fab Four”: a) the Who b) Queen c) Pink Floyd d) the Beatles 2) What city were the Beatles from? a) London b) Liverpool c) Brighton d) Oxford 3) Who was the lead singer of Queen? a) Freddie Mercury b) John Lennon c) Mick Jagger d) Robbie Williams 4) What happened to Mick Jagger in 2004? a) He left the Rolling Stones b) He became Sir Mick Jagger c) He died. -

One Direction Infection: Media Representations of Boy Bands and Their Fans
One Direction Infection: Media Representations of Boy Bands and their Fans Annie Lyons TC 660H Plan II Honors Program The University of Texas at Austin December 2020 __________________________________________ Renita Coleman Department of Journalism Supervising Professor __________________________________________ Hannah Lewis Department of Musicology Second Reader 2 ABSTRACT Author: Annie Lyons Title: One Direction Infection: Media Representations of Boy Bands and their Fans Supervising Professors: Renita Coleman, Ph.D. Hannah Lewis, Ph.D. Boy bands have long been disparaged in music journalism settings, largely in part to their close association with hordes of screaming teenage and prepubescent girls. As rock journalism evolved in the 1960s and 1970s, so did two dismissive and misogynistic stereotypes about female fans: groupies and teenyboppers (Coates, 2003). While groupies were scorned in rock circles for their perceived hypersexuality, teenyboppers, who we can consider an umbrella term including boy band fanbases, were defined by a lack of sexuality and viewed as shallow, immature and prone to hysteria, and ridiculed as hall markers of bad taste, despite being driving forces in commercial markets (Ewens, 2020; Sherman, 2020). Similarly, boy bands have been disdained for their perceived femininity and viewed as inauthentic compared to “real” artists— namely, hypermasculine male rock artists. While the boy band genre has evolved and experienced different eras, depictions of both the bands and their fans have stagnated in media, relying on these old stereotypes (Duffett, 2012). This paper aimed to investigate to what extent modern boy bands are portrayed differently from non-boy bands in music journalism through a quantitative content analysis coding articles for certain tropes and themes. -
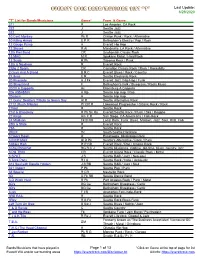
Everett Rock Band/Musician List "T" Last Update: 6/28/2020
Everett Rock Band/Musician List "T" Last Update: 6/28/2020 "T" List for Bands/Musicians Genre* From & Genre 311 R Los Angeles, CA Rock 322 J Seattle Jazz 322 J Seattle Jazz 10 Cent Monkey Pk R Clinton Punk / Rock / Alternative 10 Killing Hands E P R Bellingham's Electro / Pop / Rock 12 Gauge Pump H Everett Hip Hop 12 Stones R Al Mandeville, LA Rock / Alternative 12th Fret Band CR Snohomish Classic Rock 13 MAG M R Spokane Metal / Hard Rock 13 Scars R Pk Tacoma Rock / Punk 13th & Nowhere R Everett Rock 2 Big 2 Spank CR Carnation Classic Rock / Rock / Rockabilly 2 Guys And A Broad B R C Everett Blues / Rock / Country 2 Libras E R Seattle Electronic Rock 20 Riverside H J Fk Everett Jazz / Hip Hop / Funk 20 Sting Band F Bellingham's Folk / Bluegrass / Roots Music 20/20 A Cappella Ac Ellensburg A Cappella 206 A$$A$$IN H Rp Seattle Hip Hop / Rap 20sicem H Seattle Hip Hop 21 Guns: Seattle's Tribute to Green Day Al R Seattle Alternative Rock 2112 (Rush Tribute) Pr CR R Lakewood Progressive / Classic Rock / Rock 21feet R Seattle Rock 21st & Broadway R Pk Sk Ra Everett/Seattle Rock / Punk / Ska / Reggae 22 Kings Am F R San Diego, CA Americana / Folk-Rock 24 Madison Fk B RB Local Rock, Funk, Blues, Motown, Jazz, Soul, RnB, Folk 25th & State R Everett Rock 29A R Seattle Rock 2KLIX Hc South Seattle Hardcore 3 Doors Down R Escatawpa, Mississippi Rock 3 INCH MAX Al R Pk Seattle's Alternative / Rock / Punk 3 Miles High R P CR Everett Rock / Pop / Classic Rock 3 Play Ricochet BG B C J Seattle bluegrass, ragtime, old-time, blues, country, jazz 3 PM TRIO -

Chapter 2: Pop-Rock of the 50S and 60S
The Development of Rock & Roll by Dr. Daniel Jacobson © 2016 All Rights Reserved CHAPTER 2 POP-ROCK OF THE 50S AND 60S INTRODUCTION Softer “pop” music styles have played important roles in the development of rock, especially from c1953 to 1966 and in the early 70s. Various pop-related styles in the 50s and early 60s include: • Doo-Wop (1950s and early ‘60s; combined Pop, Gospel and soft R & B elements) • Teen Idol “Crooners” (late ‘50s/early ‘60s; Dick Clark early rock era; soft R & B) • Surf Music (late ‘50s/early ‘60s) • Brill Building/Aldon Music (‘60s pop; extension of “Tin Pan Alley” tradition) • Early Motown (early 1960s; “Soul-pop” music) I. THE CHANGING OF THE GUARD IN 50s ROCK During the years 1957 to 1961, Rock & Roll lost the impact of at least ten of its most prominent trendsetters. • March 1956: While on his way to perform for The Perry Como Show in New York City, Carl Perkins was involved in a car crash in which he suffered a fractured shoulder and skull. Perkins lost his chance for major fame, and was soon overshadowed by the rise of Elvis Presley. • October 1957: Little Richard renounced Rock and Roll for the ministry of the Seventh Day Adventist Church. • November 1957: Jerry Lee Lewis married his 13-year-old third cousin (while “forgetting” to divorce his first wife.) The scandal that followed destroyed his career. • March 1958: Elvis Presley was drafted into the army, serving in Germany until 1960. • February 1959: a small-plane crash near Fargo, North Dakota killed Buddy Holly, J.P. -

Pop and Rock
Week 9: Pop and rock 200512 Recap: use of blues scale and 3-chord progression in pop and rock , Beatles’ British invasion expanded content and harmonies for development of pop/rock music The majority of pop and rock musicians have no formal academic music education, so can’t read music/write down the notes à why improv plays such a big part in jazz and blues. Ear, skills and practical knowledge is the highest authority in the realm of popular music. Features of popular music • In pop music, all types of chord changes are allowed (unlike in strict classical music). However, stereotypical chordal progressions are often used o E.g. C, G, Am, F; or variant Am, F, C, G • Structure of AABAB or AABABCB (or variations of verse-chorus-verse-chorus) o E.g. track HELP! Using AABAB structure • Harmony in pop music is often based on triads and 7th chords o Triad à chord consisting of 3 notes o 7th chords à chord consisting of 4 notes; trial + 7th interval o Jazz also uses 9th, 11th, 13th chords • Use of drone (e.g. track Say Something having rhythmic drone all throughout song) • Ostinato is present everywhere à repetition of short phrases provides catchiness Connection between pop/rock and traditional music • Pop/rock groups often create songs themselves, unlike classical genre in an orchestral setting where the composer has created the music o Therefore pop/rock tend to have less specialization à the musician writes, entertains, arranges, etc. o Classical music: composer writes, conductor leads, each part in orchestra plays a different part that has already been dictated • Pop/rock is similar to traditional where there is a collaborative sense in writing music together o There is no barrier between performer and audience in traditional music; participation is encouraged o Similar to pop/rock music, where bands during the concert actively encourage audience to dance or sing along to the performance; not strictly divided compared to classical à only can clap during very specific times § E.g.