The Implications of Grey Seal Movements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Duckpool to Wanson Mouth
www.gov.uk/englandcoastpath England Coast Path Stretch: Marsland Mouth to Newquay Report MNQ 2: Duckpool to Wanson Mouth Part 2.1: Introduction Start Point: Duckpool (grid reference: SS 2026 1164) End Point: Wanson Mouth (grid reference: SS 1964 0094) Relevant Maps: MNQ 2a to MNQ 2g 2.1.1 This is one of a series of linked but legally separate reports published by Natural England under section 51 of the National Parks and Access to the Countryside Act 1949, which make proposals to the Secretary of State for improved public access along and to this stretch of coast between Marsland Mouth and Newquay. 2.1.2 This report covers length MNQ 2 of the stretch, which is the coast between Duckpool and Wanson Mouth. It makes free-standing statutory proposals for this part of the stretch, and seeks approval for them by the Secretary of State in their own right under section 52 of the National Parks and Access to the Countryside Act 1949. 2.1.3 The report explains how we propose to implement the England Coast Path (“the trail”) on this part of the stretch, and details the likely consequences in terms of the wider ‘Coastal Margin’ that will be created if our proposals are approved by the Secretary of State. Our report also sets out: any proposals we think are necessary for restricting or excluding coastal access rights to address particular issues, in line with the powers in the legislation; and any proposed powers for the trail to be capable of being relocated on particular sections (“roll- back”), if this proves necessary in the future because of coastal change. -

Residential Development of up to 4 Dwellings at Land Adjacent to Bingera Cottage, Madeira Drive, Widemouth Bay {220050, 102402}
Proposed Outline Planning Application for Residential Development of up to 4 Dwellings at Land adjacent to Bingera Cottage, Madeira Drive, Widemouth Bay {220050, 102402} DESIGN & ACCESS STATEMENT v1.4 prepared by: THE BAZELEY PARTNERSHIP Chartered Architects Efford Farm Business Park Bude, Cornwall EX23 8LT 01288 355557 [email protected] CONTENTS 1.0 INTRODUCTION p4 2.0 SITE HISTORY p5 3.0 AMBITIONS p7 4.0 CONTEXT APPRAISAL p8 5.0 USE & AMOUNT p12 6.0 LAYOUT p15 7.0 SCALE & MASSING p19 8.0 APPEARANCE p25 9.0 LANDSCAPING p26 A high-quality proposed residential development of 4no. dwellings distributed as an indicative mix of detached two-storey and ‘room-in-the-roof’ dwellings, each around 10.0 DRAINAGE p28 140m2 in size and with a mix of off-road and garage vehicular parking. The proposal works with the semi-rural settlement location and proposes the continuation 11.0 ECOLOGY p29 of the built form along Madeira Drive with Plots 1-3 adding an element of layering and interest to the street scene through a variation in aesthetics, ridge heights and distances from the highway. 12.0 ACCESS p30 The proposal places an emphasis on rural place-making rather than a high-density layout, with generous gardens / amenity spaces and priority given to retaining and encouraging wildlife through site-wide soft landscaping and permeable boundaries between plots. Land adj. Bingera Cottage, Widemouth Bay DESIGN & ACCESS STATEMENT THE BAZELEY PARTNERSHIP [email protected] page 2 The Surf House The Phoenix Tek-Chy Sea Quarts Sea Haze -

JNCC Coastal Directories Project Team
Coasts and seas of the United Kingdom Region 11 The Western Approaches: Falmouth Bay to Kenfig edited by J.H. Barne, C.F. Robson, S.S. Kaznowska, J.P. Doody, N.C. Davidson & A.L. Buck Joint Nature Conservation Committee Monkstone House, City Road Peterborough PE1 1JY UK ©JNCC 1996 This volume has been produced by the Coastal Directories Project of the JNCC on behalf of the project Steering Group and supported by WWF-UK. JNCC Coastal Directories Project Team Project directors Dr J.P. Doody, Dr N.C. Davidson Project management and co-ordination J.H. Barne, C.F. Robson Editing and publication S.S. Kaznowska, J.C. Brooksbank, A.L. Buck Administration & editorial assistance C.A. Smith, R. Keddie, J. Plaza, S. Palasiuk, N.M. Stevenson The project receives guidance from a Steering Group which has more than 200 members. More detailed information and advice came from the members of the Core Steering Group, which is composed as follows: Dr J.M. Baxter Scottish Natural Heritage R.J. Bleakley Department of the Environment, Northern Ireland R. Bradley The Association of Sea Fisheries Committees of England and Wales Dr J.P. Doody Joint Nature Conservation Committee B. Empson Environment Agency Dr K. Hiscock Joint Nature Conservation Committee C. Gilbert Kent County Council & National Coasts and Estuaries Advisory Group Prof. S.J. Lockwood MAFF Directorate of Fisheries Research C.R. Macduff-Duncan Esso UK (on behalf of the UK Offshore Operators Association) Dr D.J. Murison Scottish Office Agriculture, Environment & Fisheries Department Dr H.J. Prosser Welsh Office Dr J.S. -

Camelford Community Network Population 12,341
across a variety of topics. Community network figures have been built from neighbourhood data. Availability of parish data is limited. is data parish of Availability data. neighbourhood from built been have figures network Community topics. of avariety across Cornwall to compares it how highlighting (CNA) Area Network Community Camelford the for figures headline out sets document This Network Community Camelford Percentage 10 Population 0 1 2 3 4 5 6 7 8 9 0-4 (%) profile2011 Camelford Community Network population 5-9 10-14 15-19 20-24 facts and figures figures facts and 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60-64 65-69 (Census 2011) 70-74 75-79 80-84 85+ Cornwall Camelford Age September 2014 [email protected] September 2014 (LSOA). Areas Output Super Lower called sometimes and (ONS) Statistics National of the by Office defined are They them. 3,000in and people living between 1,000 have which areas geographical small are These ‘neighbourhoods’. to reference make we document this Throughout Network population growth 2001 –2011 2001 growth population Network 1,380 people (net) commute out of the network area to work. to area network the of out commute (net) people 1,380 so 10,961 is population workday The population. total Cornwall’s Network Community Camelford People growth2001-2011 population Network 10 12 20 40 60 80 00 00 0 0 0 0 0 11.6% CNA Camelford 0-4 5-9 10-14 15-19 20-24 Population 12,341 Population 25-29 30-34 35-39 has 12,341 residents; 2.3% of of 2.3% residents; 12,341 has 40-44 45-49 50-54 6.6% Cornwall 55-59 60-64 65-69 70-74 75-79 80-84 20 20 11 85+ 01 Age 1 Access to services – Housing 2 Home ownership (%) (Census 2011) 471 households live in social rented housing in the Camelford CNA which is Private Camelford CNA lower (8.9%) than the Cornwall average (12%) as shown in the chart left. -

Cornwall AONB Monitoring: Phase 2
Cornwall AONB Monitoring: Phase 2 Draft report Prepared by LUC in association with Plymouth University October 2013 Project Title: Cornwall AONB Monitoring: Phase 2 Client: Cornwall AONB Unit Version Date Version Details Prepared by Checked by Approved by Principal 0.1 09.05.13 First internal draft of the Sally Parker Diana Manson Lyndis Cole report structure 0.2 21.05.13 DM comments and SP Diana Manson Sally Parker Lyndis Cole additions Sally Parker 1.0 11.09.13 Draft final report circulated Diana Manson Sally Parker Lyndis Cole to client Sally Parker Faye Davey Maria Grant 1.1 19.09.13 Additions to draft final Diana Manson Sally Parker Lyndis Cole report Maria Grant Sally Parker 2.0 31.10.13 Final report Sally Parker Diana Manson Lyndis Cole Maria Grant J:\CURRENT PROJECTS\5600s\5683 Cornwall AONB Monitoring project Phase 2\C Project Outputs\5683_AONB Monitoring Report_20131031_V2_0.docx Cornwall AONB Monitoring: Phase 2 Draft final report Prepared by LUC in association with Plymouth University October 2013 Planning & EIA LUC BRISTOL Offices also in: Land Use Consultants Ltd Design 14 Great George Street London Registered in England Registered number: 2549296 Landscape Planning Bristol BS1 5RH Glasgow Registered Office: Landscape Management Tel:0117 929 1997 Edinburgh 43 Chalton Street Ecology Fax:0117 929 1998 London NW1 1JD LUC uses 100% recycled paper Mapping & Visualisation [email protected] FS 566056 EMS 566057 Contents 1 Introduction 1 The Cornwall AONB Monitoring Project 1 Method undertaken for Phase 2 1 Structure of this -

BOSCASTLE to WIDEMOUTH DISTRICT: North Cornwall Status
COUNTY: Cornwall SITE NAME: BOSCASTLE TO WIDEMOUTH DISTRICT: North Cornwall Status: Site of Special Scientific Interest (SSSI) notified under Section 28 of the Wildlife and Countryside Act 1981 (as amended) Local Planning Authority: Cornwall County Council, North Cornwall District Council National Grid Reference: SX 092916–SS 194018 Area: 639 (ha) 1579 (ac) Ordnance Survey Sheet 1:50,000: 190 1:10,000: SX 09 SE, SX 19 SW, NW, NE, SS 10 SE Date Notified (Under 1949 Act): 1972 Date of Last Revision: – Date Notified (Under 1981 Act): 1990 Date of Last Revision: – Other Information: Within an Area of Outstanding Natural Beauty and North Cornwall Heritage Coast; part owned by the National Trust; includes 5 Geological Conservation Review sites and is noted in “A Nature Conservation Review” Ed. D A Ratcliffe (Cambridge University Press 1977). Site amended by extensions and deletions. Description and Reasons for Notification: This site lies on the North Cornwall coast and comprises a 12 mile section of cliffs and coastal habitats between Boscastle and Widemouth. The cliffs exhibit classic geological exposures of Namurian rocks and Variscan structures; the outstanding biological interest includes the unique Dizzard Oak woodland, maritime heaths and intertidal zones. Five Geological Conservation Review Localities occur within the site: 1. Widemouth to Crackington – This site is comprised of extensive coastal exposures, where the typically developed basinal Namurian of south-west England is clearly exposed. The entire Namurian represented by the Crackington formation is visible within the site, and the presence of rare goniatites has been vital in unravelling the complicated local stratigraphy. The section provides an excellent display of the sedimentary features associated with shallow water turbidites, and is of considerable interest for its spectacular structural features. -

2 Widemouth Bay
Appendix C Baseline Report Stage 1 Report: Widemouth Bay Cornwall Beach & Dune Management Plans Prepared for Cornwall Council 9 July 2015 Ash House Falcon Road Sowton Exeter Devon EX2 7LB Contents Section Page 1 Introduction ............................................................................................................................ 1 1.1 Background ......................................................................................................................... 1 1.2 Project aim, objectives and approach ................................................................................ 1 1.3 About this document .......................................................................................................... 4 2 Site Visit Report ....................................................................................................................... 5 2.1 Attendees ............................................................................................................................ 5 2.2 Site Visit Record .................................................................................................................. 5 2.3 Thoughts on Potential Management Solutions for Consideration ..................................... 6 2.4 Data Sources ....................................................................................................................... 6 2.5 Photos ................................................................................................................................. 7 3 Environmental -

North Cornwall & North Devon
Archaeology, Art & Coastal Heritage: Tools to Support Coastal Management (Arch-Manche) _____________________________________________________________________________________________ CASE STUDY 3G – NORTH CORNWALL & NORTH DEVON Case study area: North Cornwall and North Devon, UK. Main geomorphological types: Hard cliffs, rocky outcrops, sandy beaches. Main coastal change processes: Coastal erosion, beach change, some cliff instability, low lying areas vulnerable to flooding. Primary resources used: Archaeology. Summary: The high cliffs, interspersed with small natural harbours and sandy beaches face the full force of the Atlantic Ocean. The cliffs consist of relatively hard geology but are vulnerable to landslides. Although few major archaeological studies have been undertaken in this area, there are extensive prehistoric landscapes surviving. Knowledge of the heritage resource clearly demonstrates changes in relation to erosion and changes in sedimentation. Recommendations: Coastal managers should use archaeological and palaeoenvironmental resources to understand long term changes, in particular where humanly-made structures (such as Bude breakwater) have influenced the sediment regime. Extensive Bronze Age peats buried under the coast provide opportunities for detailed modelling of change. Coastal managers face an ongoing battle to moderate impacts from the sea in the face of a changing climate and pressures from human use of the coastal zone. The challenges that lie ahead are forecast to increase while resources are being forced to go further. This case study report is part of the Arch-Manche project, which quantifies the value of under- used coastal indicators that can be applied as tools to inform long term patterns of coastal change. In addition, it provides instruments to communicate past change effectively, model areas under threat and interpret progressive coastal trends. -

Local Environment Agency Plan
local environment agency plan NORTH CORNWALL CONSULTATION REPORT DECEMBER 1997 BUDE BO D M IN NEWQUAY YOUR VIEWS This Consultation Report is our initial view of the issues facing the catchment. Public consultation allows people who live in or use the catchment to have a say in the development of our plans and work programmes. We welcome your ideas on the future management of this catchment: • Have w e identified all the issues? • Have we identified all the options for solutions? • Have you any comments on the issues and options listed? • Do you have any other information or views that you wish to bring to our attention? This is your opportunity to influence our future plans. We look forward to hearing from you. Geoff Boyd Area Manager, Cornwall E n v ir o n m e n t Ag e n c y Please send your comments by 9 March 1998, preferably by writing to: NATIONAL LIBRARY & INFORMATION SERVICE Team Leader, LEAPs Environment Agency Sir |ohn Moore House SOUTH WEST REGION Victoria Square B o d m in Manley House, Kestrel Way, Exeter EX2 7LQ Cornwall PL31 1EB Tel: 01208 78301 Fax: 01208 78321 Environment Agency Copyright Waiver This report is intended to be used widely, and may be quoted, copied or reproduced in any way, provided that the extracts are not quoted out of context and that due acknowledgement is given to the Environment Agency. Published December 1997. 2 North Cornwall LEAP Consultation Report Ef\ - WW' U ^ / '03 \J The North Cornwall Catchment is an area of great diversity and outstanding beauty. -

Cornwall and Isles of Scilly SMP2 SEA Soep 181110 JD
Statement of Environmental Particulars for the Cornwall and Isles of Scilly SMP2 Issued October 2010 Cornwall and Isles of Scilly SMP2 SEA 1 Statement of Environmental Particulars Contents Section 1 – Introduction...................................................................................................3 Purpose of this SEA Statement of Environmental Particulars ......................................3 Section 2 – Background ..................................................................................................5 The Cornwall and Isles of Scilly Shoreline Management Plan 2...................................5 Strategic Environmental Assessment...........................................................................8 Section 3 - Alternatives ...................................................................................................9 Section 4 – Integration of Environmental Considerations ..............................................10 The Scoping Report (July 2009) ................................................................................10 The Environmental Report (March 2010) ...................................................................11 Section 5 – Influence of the Environmental Report........................................................73 Section 6 – Consultation ...............................................................................................74 Section 7 – Environmental Monitoring Measures for the Implementation of this SMP2..78 Effects on the integrity of international sites...............................................................79 -
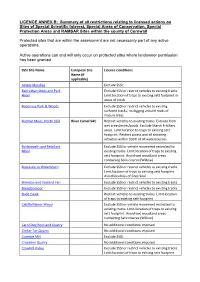
Summary of All Restrictions Relating to Licensed Actions on Sites of Special
LICENCE ANNEX B: Summary of all restrictions relating to licensed actions on Sites of Special Scientific Interest, Special Areas of Conservation, Special Protection Areas and RAMSAR Sites within the county of Cornwall Protected sites that are within the assessment are not necessarily part of any active operations. Active operations can and will only occur on protected sites where landowner permission has been granted. SSSI Site Name European Site Licence Conditions Name (if applicable) Amble Marshes Exclude SSSI. Bedruthan Steps and Park Exclude SSSI or restrict vehicles to existing tracks. Head Limit location of traps to existing sett footprint or areas of scrub Boconnoc Park & Woods Exclude SSSI or restrict vehicles to existing surfaced tracks; no digging around roots of mature trees Bodmin Moor, North SSSI River Camel SAC Restrict vehicles to existing tracks. Exclude from wet areas/mires/pools. Exclude Marsh Fritillary areas. Limit location to traps to existing sett footprint. Restrict access and all shooting activities within 100m of all watercourses. Borlasevath and Retallack Exclude SSSI or vehicle movement restricted to Moor existing tracks. Limit location of traps to existing sett footprint. Avoid wet woodland areas containing Salix cinerea (Willow) Boscastle to Widemouth Exclude SSSI or restrict vehicles to existing tracks. Limit location of traps to existing sett footprint. Avoid localities of Grey Seal. Brendon and Vealand Fen Exclude SSSI or restrict vehicles to existing tracks Brendonmoor Exclude SSSI or restrict vehicles to existing tracks Bude Coast Restrict vehicles to existing tracks. Limit location of traps to existing sett footprint. Cabilla Manor Wood Exclude SSSI or vehicle movement restricted to existing tracks. -

PDZ: 15 Pentire Point to Wanson Mouth Management Area 37
PDZ: 15 Pentire Point to Wanson Management Area 37 Mouth Management Area 38 Trebarwith Strand Pentire Point to Wanson Mouth This section of coastline faces generally north to north-west and its exposed nature is reflected in the rugged cliffs, stormy seas, stunted and wind blown trees, and isolated settlements which have grown up clustered in the shelter of river valleys. Pocket beaches are found occasionally along the cliffed coastline where steep valleys have formed allowing watercourses to reach the sea. Geographically it is one of the largest sections of coastline dealt with within this SMP, covering around 50km of exposed frontage but it is one of the most sparsely populated. Agriculture forms the bulk of the cliff top land use with small scale fishing fleets still operating from some of the sheltered natural harbours. Cornwall and Isles of Scilly SMP2 Final Report Chapter 4 PDZ15 1 February 2011 Policy Development Zone 15 – Pentire Point to Wanson Mouth Cornwall and Isles of Scilly SMP2 Final Report Chapter 4 PDZ15 2 February 2011 General Description Built Environment The character of the area is one of wild and unspoilt coastline with isolated communities existing within a framework of cliff top agriculture, small scale fishing operations and low key tourism at discrete locations such as Port Isaac and Boscastle. Port Isaac, Tintagel and Boscastle represent the key settlements in terms of population and visitor numbers but of these only Port Isaac has a significant part of the settlement in close proximity to mean high water. Three much smaller settlements exist at Port Quin, Port Gaverne and Crackington Haven (pictured right) and these all Crackington Haven have a limited number of properties close to the waters edge.