The Dominant Eye: Dominant for Parvo- but Not for Magno-Biased Stimuli?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
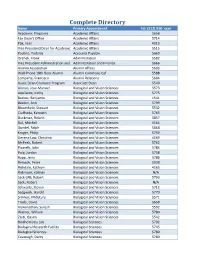
Complete Directory
Complete Directory Name Primary Appointment Tel: (212) 938- xxxx Academic Programs Academic Affairs 5658 Fax Dean's Office Academic Affairs 5714 Pak, Jean Academic Affairs 4313 Vice President/Dean for Academic Academic Affairs 5515 Paulino, Yodania Accounts Payable 5669 Orehek, Frank Administration 5582 Vice President Administration and Administration and Finance 5666 Alumni Association Alumni Affairs 5603 Wall Phone 18th floor Alumni Alumni Commons Caf 5588 Lomparte, Francisco Alumni Relations 5604 Assoc Dean Graduate Program Associate Dean 5540 Alonso, Jose-Manuel Biological and Vision Sciences 5573 Aquilante, Kathy Biological and Vision Sciences 5775 Backus, Benjamin Biological and Vision Sciences 1541 Beaton, Ann Biological and Vision Sciences 5799 Bloomfield, Stewart Biological and Vision Sciences 5532 Ciuffreda, Kenneth Biological and Vision Sciences 5765 Duckman, Robert Biological and Vision Sciences 5857 Dul, Mitchell Biological and Vision Sciences 4164 Gundel, Ralph Biological and Vision Sciences 5868 Kruger, Philip Biological and Vision Sciences 5759 Llerena-Law, Christina Biological and Vision Sciences 4169 McPeek, Robert Biological and Vision Sciences 5762 Picarelli, John Biological and Vision Sciences 5784 Pola, Jordan Biological and Vision Sciences 5758 Rapp, Jerry Biological and Vision Sciences 5786 Reinach, Peter Biological and Vision Sciences 5938 Richdale, Kathryn Biological and Vision Sciences 4165 Rubinson, Kalman Biological and Vision Sciences N/A Sack LAB, Robert Biological and Vision Sciences 5793 Sack, Robert Biological -

AGE Qualitative Summary
AGE Qualitative Summary Age Gender Race 16 Male White (not Hispanic) 16 Male Black or African American (not Hispanic) 17 Male Black or African American (not Hispanic) 18 Female Black or African American (not Hispanic) 18 Male White (not Hispanic) 18 Malel Blacklk or Africanf American (not Hispanic) 18 Female Black or African American (not Hispanic) 18 Female White (not Hispanic) 18 Female Asian, Asian Indian, or Pacific Islander 18 Male Asian, Asian Indian, or Pacific Islander 18 Female White (not Hispanic) 18 Female White (not Hispanic) 18 Female Black or African American (not Hispanic) 18 Male White (not Hispanic) 19 Male Hispanic (unspecified) 19 Female White (not Hispanic) 19 Female Asian, Asian Indian, or Pacific Islander 19 Male Asian, Asian Indian, or Pacific Islander 19 Male Asian, Asian Indian, or Pacific Islander 19 Female Native American or Alaskan Native 19 Female White (p(not Hispanic)) 19 Male Hispanic (unspecified) 19 Female Hispanic (unspecified) 19 Female White (not Hispanic) 19 Female White (not Hispanic) 19 Male Hispanic/Latino – White 19 Male Hispanic/Latino – White 19 Male Native American or Alaskan Native 19 Female Other 19 Male Hispanic/Latino – White 19 Male Asian, Asian Indian, or Pacific Islander 20 Female White (not Hispanic) 20 Female Other 20 Female Black or African American (not Hispanic) 20 Male Other 20 Male Native American or Alaskan Native 21 Female Don’t want to respond 21 Female White (not Hispanic) 21 Female White (not Hispanic) 21 Male Asian, Asian Indian, or Pacific Islander 21 Female White (not -

10 Pre-Game Mistakes
10 “Deadly” Mistakes Hockey Players Make – Page 1 Peak Performance Sports Special Report The 10 “Deadly” Mistakes Hockey Players Make With Their Pregame Attitude What every player and sports parent needs to learn to improve athletes’ mental game Patrick J. Cohn, Ph.D. Mental Game Coach Peak Performance Sports, LLC http://www.peaksports.com/mental _________________________________________________________________ Copyright © 2008 by Peak Performance Sports, LLC. 10 “Deadly” Mistakes Hockey Players Make – Page 2 TERMS OF USE You may freely distribute this Peak Performance E‐booklet to teammates, friends, and coaches, as long as the entire E‐booklet remains intact, as is (without any modification) including logo, contact data, terms of use and copyright information. The information contained in this document represents the current view of Peak Performance Sports, LLC on the issues discussed as of the date of publication. Peaksports cannot guarantee the accuracy of any information presented after the date of publication. This Peak Performance E‐booklet is for informational purposes only. Peaksports MAKES NO WARRANTIES, EXPRESS, IMPLIED OR STATUTORY, AS TO THE INFORMATION IN THIS DOCUMENT. Complying with all applicable copyright laws is the responsibility of the user. Without limiting the rights under copyright, no part of this document may be reproduced, modified or distributed for profit in any form or by any means (electronic, mechanical, photocopying, recording, or otherwise) without the express written permission of Peaksports and Dr. Patrick Cohn. BY PROCEEDING WITH THIS PEAK PERFORMANCE E‐BOOKLET, YOU AGREE TO ABIDE BY THE ABOVE TERMS AND CONDITIONS WITHOUT LIMITATION. Copyright © 2008 by Peak Performance Sports, LLC. & Patrick J. -

Movielistings
The Goodland Star-News / Friday, July 6, 2007 5 Like puzzles? Then you’ll love sudoku. This mind-bending puzzle will have FUN BY THE NUMBERS you hooked from the moment you square off, so sharpen your pencil and put your sudoku savvy to the test! Here’s How It Works: Sudoku puzzles are formatted as a 9x9 grid, broken down into nine 3x3 boxes. To solve a sudoku, the numbers 1 through 9 must fill each row, col- umn and box. Each number can appear only once in each row, column and box. You can figure out the order in which the numbers will appear by using the numeric clues already provided in the boxes. The more numbers you name, the easier it gets to solve the puzzle! ANSWER TO TUESDAY’S SATURDAY EVENING JULY 7, 2007 SUNDAY EVENING JULY 8, 2007 6PM 6:30 7PM 7:30 8PM 8:30 9PM 9:30 10PM 10:30 6PM 6:30 7PM 7:30 8PM 8:30 9PM 9:30 10PM 10:30 E S E = Eagle Cable S = S&T Telephone E S E = Eagle Cable S = S&T Telephone Dog Bounty Dog Bounty Family (TV14) Family Jewels Harry Potter: The Hidden Gene Simmons Family Dog Bounty Dog Bounty Flip This House: Con- Flip This House: Little House Confession Confessions Justice: The Brit and the Flip This House: Con- 36 47 A&E 36 47 A&E demned! (TV G) (R) of Horrors (R) (TV14) (R) Bodybuilder demned! (TV G) (R) (R) (R) (N) (R) Secrets (N) (HD) Jewels (TV14) (R) (R) (R) Extreme Makeover: Home Desperate Housewives: Like (:01) Brothers & Sisters KAKE News (:35) KAKE (:05) Lawyer (:35) Paid “Wonderful World of Disney: Monsters, Inc.” (‘01, America’s Funniest Home KAKE News (:35) American Idol Re- (:35) Enter- 4 6 ABC 4 6 ABC Animated) aaa (G) (R) (HD) Videos (TVPG) (R) at 10 wind: CBS 8 to 7 tainment Edition (R) It Was (R) (HD) (TVPG) (R) (HD) at 10 Sports on Program Chased by Sea Monsters Most risky sea predators to Giant Monsters (TV G) Mutual of Omaha’s Wild Chased by Sea Monsters (5:00) “Shiloh” (‘97, Family) In Search of the King Co- Wild Kingdom: King Cobra “Shiloh” (‘97, Family) Michael Moriarty. -

Briarpatch” to Film in New Mexico
Michelle Lujan Grisham Governor Alicia J. Keyes Cabinet Secretary Todd Christensen Director FOR IMMEDIATE RELEASE: Bruce Krasnow June 17, 2019 (505) 827-0226, cell: (505) 795-0119 [email protected] The New Mexico Film Office Announces USA Network’s “Briarpatch” to film in New Mexico SANTA FE, N.M. The New Mexico State Film Office today announced that the USA Network television series, “Briarpatch” produced by UCP (Universal Content Productions) and Paramount Television will begin principal photography mid-June through the end of September in Albuquerque. Starring Rosario Dawson, who will also serve as a producer, the production will employ approximately 100 New Mexico crew members and 250-350 New Mexico background talent per episode. “Briarpatch” follows Allegra Dill (Dawson), a dogged investigator returning to her border- town Texas home after her sister is murdered. What begins as a search for a killer turns into an all-consuming fight to bring her corrupt hometown to its knees. The season celebrates the beloved genres represented by Thomas’ book -- a stylish blend of crime and pulp fiction -- while updating his sense of fun, danger and place for a new generation. Based on the Ross Thomas novel of the same name, “Briarpatch” is written for television by Andy Greenwald, who will executive produce along with “Mr. Robot” creator Sam Esmail through his production company Esmail Corp and Anonymous Content’s Chad Hamilton. The series also stars Jay R. Ferguson (“Mad Men,” “The Romanoffs”), Brian Geraghty (“Chicago P.D.,” “Ray Donovan”) and Edi Gathegi (“StartUp”). ### Visit the New Mexico Film Office online at nmfilm.com About Universal Content Productions (UCP) UCP is a premium content studio that operates with a highly curated indie sensibility, while simultaneously leveraging the power and scale of NBCUniversal. -
Today Friday Daytime April 17 Cs – Charter Spectrum Dtv – Directv D – Dish Movies Sports Kids
TVTODAY FRIDAY DAYTIME APRIL 17 CS – CHARTER SPECTRUM DTV – DIRECTV D – DISH MOVIES SPORTS KIDS . Tonight's CS DTV D 8 AM 8:30 9 AM 9:30 10 AM 10:30 11 AM 11:30 12 PM 12:30 1 PM 1:30 2 PM 2:30 3 PM 3:30 4 PM 4:30 BROADCAST CHANNELS best bets KTLM 2 40 40 (7:00) Un nuevo dia (N) Dueños Decision Perro Noticias Noticias En CasaSuelta la sopa Al rojo vivo (N) Al Rojo Vivo (N) KGBT 4 4 4 (7:00) Morning (N) Daytime Price Right Young & R. (N) News B & B The Talk (N) Make a Deal Jeop.Jeop (N) Judy Judy KRGV 5 5 5 (7:00) GMA (N) Live The View Pandemic News (N) General Hospital Wendy Williams Feud Feud Ellen DeGeneres FOX 6 2 2 Minute Healthy H.Bench H.Bench The Real Friends Friends TMZExtra People Court (N) Kelly Clarkson The Dr. Oz Show Dateline XHAB 7 Agenda Pública Matutino Express Ciclo Festival de Gala Noticias Al Dia Onda Juvenil A Las 3 XERV 19 Al Aire Paola (N) Hoy (N) Cuéntamelo Ya!Juego Estrellas Que Pobres TanDestilando amor KTVF 20 22 Per Inq. Minute News News Today (N) Today III (N) Live Hoda - Jenna (N) Modern Mom Days. Lives (N) KVEO 8 23 23 (7:00) Today (N) Today III (N) Hoda - Jenna (N) Rachael Ray Days. Lives (N) Daily Daily Mel Robbins The Doctors Dr. Phil KLUJ 9 44 Creflo J.Hagee Osteen Intend Copelnd King FurtickR.Morris Life BetterTo Life CBD1 The 700 Club J.Hagee HopePraise KNVO 3 48 48 (7:00) Despierta America Te perdone Dios Noticier Noticier Como dice dichoMe Declaro C Univision Presen Gordo y flaca KMBH 10 60 60 Xavier Luna D.Tiger Clifford Sesame PinkaPet DinoT CatHat SesameSplashB. -

Mr. Robot's Elliot Alderson – the Hacker As a Vigilante Superhero For
Faculteit Letteren & Wijsbegeerte Mr. Robot’s Elliot Alderson – The Hacker as a Vigilante Superhero for the 21st Century Characterization Through the Myth of the Superhero Paper submitted in partial fulfilment of the requirements for the degree of “Master in de Vergelijkende Modene Letterkunde” 2016-2017 Promotor prof. dr. Gert Buelens Department of Literature Acknowledgements I would like to thank Prof. Dr. Gert Buelens for giving me the opportunity to write my thesis on a subject that genuinely interests me. I could not have completed this research without his help and guidance. I am also grateful to my friends and family for supporting me throughout the process of thesis writing. Two people, in particular, really came through for me: my sister, who was always there for me with moral support, snacks and beverages, and my best friend, who let me stay at his place while he was abroad – taking a well-deserved vacation. This project goes out to you. 2 List of Figures Fig. 1-2. “eps2.0_unm4sk-pt2.tc” Mr. Robot, season 2, episode 2, USA network, Fig. 4-6. 13 Jul. 2016. Telenet Play More, www.yeloplay.be/series/drama/mr- robot/seizoen-2/aflevering-2 Fig. 3. “eps1.0_hellofriend.mov”, Mr. Robot – Season 1, written by Sam Esmail, Fig. 12-13. directed by Niels Arden Oplev, USA Network, 2016. DVD. Fig. 7. Chatman, Seymour. Coming to Terms: The Rhetoric of Narrative in Fiction and Film. Ithaca: Cornell University Press, 1990. PDF. Fig. 8-9. “eps1.7_wh1ter0se.m4v” Mr. Robot – Season 1, written by Randolph Leon, directed by Christopher Schrewe, USA Network, 2016. -

Movies and Mental Illness Using Films to Understand Psychopathology 3Rd Revised and Expanded Edition 2010, Xii + 340 Pages ISBN: 978-0-88937-371-6, US $49.00
New Resources for Clinicians Visit www.hogrefe.com for • Free sample chapters • Full tables of contents • Secure online ordering • Examination copies for teachers • Many other titles available Danny Wedding, Mary Ann Boyd, Ryan M. Niemiec NEW EDITION! Movies and Mental Illness Using Films to Understand Psychopathology 3rd revised and expanded edition 2010, xii + 340 pages ISBN: 978-0-88937-371-6, US $49.00 The popular and critically acclaimed teaching tool - movies as an aid to learning about mental illness - has just got even better! Now with even more practical features and expanded contents: full film index, “Authors’ Picks”, sample syllabus, more international films. Films are a powerful medium for teaching students of psychology, social work, medicine, nursing, counseling, and even literature or media studies about mental illness and psychopathology. Movies and Mental Illness, now available in an updated edition, has established a great reputation as an enjoyable and highly memorable supplementary teaching tool for abnormal psychology classes. Written by experienced clinicians and teachers, who are themselves movie aficionados, this book is superb not just for psychology or media studies classes, but also for anyone interested in the portrayal of mental health issues in movies. The core clinical chapters each use a fabricated case history and Mini-Mental State Examination along with synopses and scenes from one or two specific, often well-known “A classic resource and an authoritative guide… Like the very movies it films to explain, teach, and encourage discussion recommends, [this book] is a powerful medium for teaching students, about the most important disorders encountered in engaging patients, and educating the public. -

Psych: Mind Over Magic Free
FREE PSYCH: MIND OVER MAGIC PDF William Rabkin | 275 pages | 03 Sep 2009 | Penguin Putnam Inc | 9780451227447 | English | New York, United States Mind Over Magic (Psych, #2) by William Rabkin Goodreads helps you keep track of books you want to read. Want to Read saving…. Want to Read Currently Reading Read. Other editions. Enlarge cover. Error rating book. Refresh and try again. Open Preview See a Problem? Details if other :. Thanks for telling us about the problem. Return to Book Page. Based on the hit usa network series. Shawn Spencer has convinced everyone he's psychic. Now, he's either going to clean up- or be found out. Murder Psych: Mind Over Magic Magic are all in the mind When a case takes Shawn and Gus into an exclusive club for professional magicians, they're treated to a private show by the hottest act on the Vegas Strip, "Martian Magician" P'tol P'kah. But whe Based on Psych: Mind Over Magic hit usa network series. But when the wizard seemingly dissolves in a tank of water, he never rematerializes. And in his place there's a corpse in a three piece suit and a bowler hat. Eager to keep his golden boy untarnished, the magician's manager hires Shawn and Gus to uncover the identity of the dead man and find out what happened to P'tol P'kah. But to do so, the pair will have to pose as a new mentalist act, and go Psych: Mind Over Magic in a world populated by magicians, mystics, Martians-and one murderer Get A Copy. -

1 CURRICULUM VITA Donny Newsome, Phd, BCBA
W.D. Newsome, PhD 1 Jan., 2018 CURRICULUM VITA Donny Newsome, PhD, BCBA-D Fit Learning™, Reno, NV - (775) 826 3111 - [email protected] SUMMARY Dr. Newsome earned his bachelor’s degree from the University of Mississippi and his Doctorate in Psychology from the University of Nevada, Reno. He is a Board Certified Behavior Analyst (BCBA- D) and Founding Director of Fit Learning™ where he has developed a growing global network of affiliated learning laboratories. Dr. Newsome is currently serving as Past-President of the Standard Celeration Society (SCS) and President of the Academic Access Initiative, a non-profit for underserved student populations. His research and service initiatives have focused on organizational behavior and educational technologies across various business sectors including public utilities, universities, school districts, Fortune 100 companies and community service providers. The overarching value guiding Dr. Newsome’s work is contributing to the transformation of human well being through the science of teaching and learning. EDUCATION University of Mississippi B.A. in Psychology, minor in English,2006 University of Nevada, Reno M.A. in Psychology, 2008 Thesis: “An investigation of efficiency and preference for supplemental learning modules in online instruction.” – Conducted in collaboration with Washoe County School District University of Nevada, Reno Ph.D. in Psychology, 2012 (August) Dissertation: “Toward the prediction and influence of efficient driving behavior” – Conducted in collaboration with the Truckee Meadows Water Authority, Washoe County School District, and civilian drivers in the Reno area. HONORS/AWARDS • Dean’s List (Fall 2003-2004) University of Mississippi • Chancellor’s Honor Roll (Fall 2004-2005) University of Mississippi • Chancellor’s Honor Roll (Spring 2004-2005) University of Mississippi • Wilson Scholarship Recipient (2010) University of Nevada, Reno • Innovation in Translation for Sustainability Award (2012) NORC at the University of Chicago W.D. -

Planning of Saccadic Eye Movements
Psychological Research (2003) 67: 10–21 DOI 10.1007/s00426-002-0094-5 ORIGINAL ARTICLE Ju¨ri Allik Æ Mai Toom Æ Aavo Luuk Planning of saccadic eye movements Received: 9 July 2001 / Accepted: 18 March 2002 / Published online: 21 September 2002 Ó Springer-Verlag 2002 Abstract Most theories of the programming of saccadic completely specified before the movement begins. Di- eye movements (SEM) agree that direction and ampli- rection and amplitude make up a minimal set of inde- tude are the two basic dimensions that are under control pendent dimensions whose values determine the when an intended movement is planned. But they dis- identities of all possible movements. One question that agree over whether these two basic parameters are remains disputed is whether these two basic parameters specified separately or in conjunction. We measured of SEM, direction and amplitude, are specified sepa- saccadic reaction time (SRT) in a situation where in- rately or jointly in a unitary fashion. Neurophysiological formation about amplitude and direction of the required explanations are usually inclined towards holistic models movement became available at different moments in without any distinct computation of direction and am- time. The delivery of information about either direction plitude values (Sparks, 1988). Neurons in the frontal eye or amplitude prior to another reduced duration of SRT fields and in the intermediate and deep layers of the demonstrated that direction and amplitude were speci- superior colliculus, which activates immediately before fied separately rather than in conjunction or in a fixed the onset of a SEM (Robinson, 1972; Sparks, 1978; Lee, serial order. -

Psychology (PSYCH) 1
Psychology (PSYCH) 1 PSYCH 205 — EXPLORING RESEARCH IN PSYCHOLOGY PSYCHOLOGY (PSYCH) 1 credit. Focuses on a wide range of research in psychology at UW-Madison, PSYCH/ASIAN/COUN PSY/ED PSYCH 120 — THE ART AND SCIENCE OF such as child development, clinical psychology, perception, biological HUMAN FLOURISHING psychology, cognition, and psychological neuroscience. Enroll Info: None 3 credits. Requisites: PSYCH 202 Course Designation: Breadth - Social Science Explore perspectives related to human flourishing from the sciences Level - Intermediate and humanities; investigate themes such as transformation, resilience, L&S Credit - Counts as Liberal Arts and Science credit in L&S compassion, diversity, gratitude, community; expand self-awareness, Repeatable for Credit: No enhanced social connectivity, and ability to change; formulate a sense Last Taught: Spring 2021 of what it means to lead a flourishing life that sustains meaningful and fulfilling engagement with studies, relationships, community, and career. PSYCH 210 — BASIC STATISTICS FOR PSYCHOLOGY Enroll Info: None 3 credits. Requisites: None Measures of central tendency, variability; probability, sampling Course Designation: Breadth - Either Humanities or Social Science distributions; hypothesis testing, confidence intervals; t-tests; Chi- Level - Elementary square; regression and correlation (linear) and introduction to analysis L&S Credit - Counts as Liberal Arts and Science credit in L&S of variance (1-way). Enroll Info: Satisfied Quantitative Reasoning (QR) A Repeatable for Credit: No requirement; PSYCH 201 or 202 or 281 or concurrent enrollment Last Taught: Fall 2020 Requisites: Satisfied Quantitative Reasoning (QR) A requirement and PSYCH/SOC 160 — HUMAN SEXUALITY: SOCIAL AND PSYCHOLOGICAL PSYCH 201, 202, or 281 or concurrent enrollment ISSUES Course Designation: Gen Ed - Quantitative Reasoning Part B 3-4 credits.