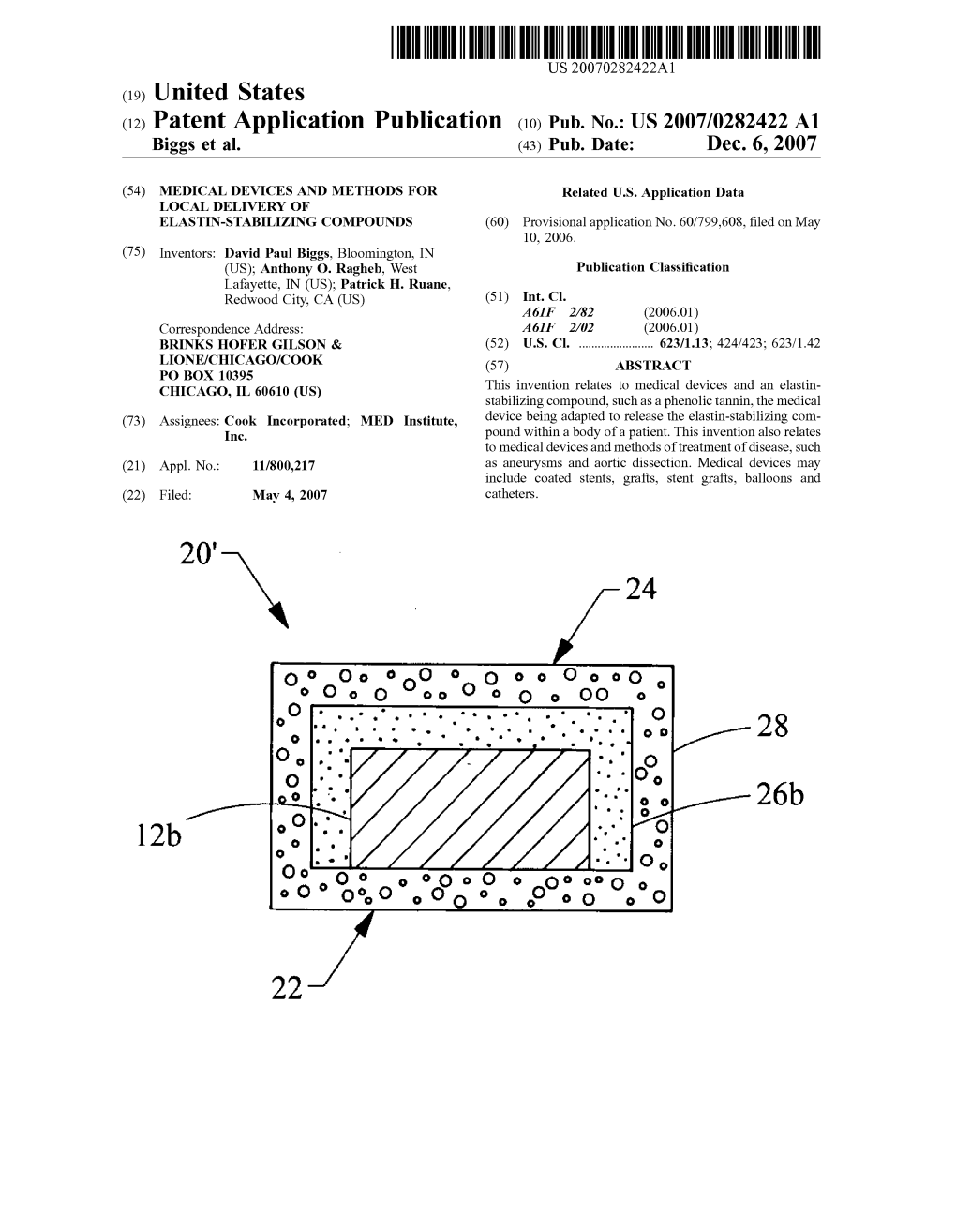
US 2007/0282422 A1 12B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wo 2007/133479 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date (10) International Publication Number 22 November 2007 (22.11.2007) PCT WO 2007/133479 A2 (51) International Patent Classification: Not classified (74) Agent: GODLEWSKI, Richard, J.; P.O. Box 2269, Bloomington, IN 47402-2269 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US2007/0 10828 kind of national protection available): AE, AG, AL, AM, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, (22) International Filing Date: 4 May 2007 (04.05.2007) CN, CO, CR, CU, CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, (25) Filing Language: English IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, MX, (26) Publication Language: English MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, TJ, TM, (30) Priority Data: TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW 60/799,608 10 May 2006 (10.05.2006) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicants (for all designated States except US): COOK GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, INCORPORATED [US/US]; 750 North Daniel's Way, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), P.O. -

Walnut Polyphenol
ORYZA OIL & FAT CHEMICAL CO., L TD. WALNUT POLYPHENOL Hepatoprotective & Anti-oxidative Extract For Metabolic Syndrome ■ WALNUT POLYPHENOL-P10,P30 (Powder,Food Grade) ■ WALNUT POLYPHENOL-WSP10 (Water-soluble Powder,Food Grade) ■ WALNUT POLYPHENOL-PC10,PC30 (Powder,Cosmetic Grade) ■ WALNUT POLYPHENOL-WSPC10 (Water-soluble Powder,Cosmetic Grade) ■ WALNUT POLYPHENOL-LC (Water-soluble Liquid,Cosmetic Grade) ■ WALNUT SEED OIL (Oil,Food & Cosmetic Grade) ORYZA OIL & FAT CHEMICAL CO., LTD ver. 1.0 HS WALNUT POLYPHENOL ver.1.0 HS WALNUT POLYPHENOL Hepatoprotective & Anti-oxidative Extract For Metabolic Syndrome 1. Introduction Recently, there is an increased awareness on metabolic syndrome – a condition characterized by a group of metabolic risk factors in one person. They include abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, insulin resistance, prothrombotic state & proinflammatory state. The dominant underlying risk factors appear to be abdominal obesity and insulin resistance. In addition, non-alcoholic fatty liver disease (NAFLD) is the most commonly associated “liver” manifestation of metabolic syndrome which can progress to advance liver disease (e.g. cirrhosis) with associated morbidity and mortality. Lifestyle therapies such as weight loss significantly improve all aspects of metabolic syndrome, as well as reducing progression of NAFLD and cardiovascular mortality. Walnut (Juglans regia L. seed) is one the most popular nuts consumed in the world. It is loaded in polyunsaturated fatty acids – linoleic acid (LA), oleic acid and α-linolenic acid (ALA), an ω3 fatty acid. It has been used since ancient times and epidemiological studies have revealed that incorporating walnuts in a healthy diet reduces the risk of cardiovascular diseases. Recent investigations reported that walnut diet improves the function of blood vessels and lower serum cholesterol. -

Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects
S310 DOI 10.1002/mnfr.200900039 Mol. Nutr. Food Res. 2009, 53, S310 – S329 Review Tannins: Current knowledge of food sources, intake, bioavailability and biological effects Jos Serrano1, Riitta Puupponen-Pimi2, Andreas Dauer3, Anna-Marja Aura2 and Fulgencio Saura-Calixto4 1 Universidad Complutense de Madrid, Depto. Nutricin y Bromatologa I, Madrid, Spain 2 VTT Technical Research Center of Finland 3 Hexal AG, Holzkirchen, Germany 4 Consejo Superior de Investigaciones Cientficas, Instituto del Frio, Depto. Metabolismo y Nutricin, Madrid, Spain Tannins are a unique group of phenolic metabolites with molecular weights between 500 and 30000 Da, which are widely distributed in almost all plant foods and beverages. Proanthocyanidins and hydrolysable tannins are the two major groups of these bioactive compounds, but complex tannins containing structural elements of both groups and specific tannins in marine brown algae have also been described. Most literature data on food tannins refer only to oligomeric compounds that are extracted with aqueous-organic solvents, but a significant number of non-extractable tannins are usu- ally not mentioned in the literature. The biological effects of tannins usually depend on their grade of polymerisation and solubility. Highly polymerised tannins exhibit low bioaccessibility in the small intestine and low fermentability by colonic microflora. This review summarises a new approach to analysis of extractable and non-extractable tannins, major food sources, and effects of storage and processing on tannin content and bioavailability. Biological properties such as antioxidant, antimicro- bial and antiviral effects are also described. In addition, the role of tannins in diabetes mellitus has been discussed. Keywords: Bioavailability / Diet / Hydrolysable tannins / Proanthocyanidins / Tannins / Received: November 27, 2007; revised: January 25, 2009; accepted: February 9, 2009 1 Introduction weight having the ability to complex strongly with carbohy- drates and proteins [9]. -

Chemistry and Pharmacology of Kinkéliba (Combretum
CHEMISTRY AND PHARMACOLOGY OF KINKÉLIBA (COMBRETUM MICRANTHUM), A WEST AFRICAN MEDICINAL PLANT By CARA RENAE WELCH A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Medicinal Chemistry written under the direction of Dr. James E. Simon and approved by ______________________________ ______________________________ ______________________________ ______________________________ New Brunswick, New Jersey January, 2010 ABSTRACT OF THE DISSERTATION Chemistry and Pharmacology of Kinkéliba (Combretum micranthum), a West African Medicinal Plant by CARA RENAE WELCH Dissertation Director: James E. Simon Kinkéliba (Combretum micranthum, Fam. Combretaceae) is an undomesticated shrub species of western Africa and is one of the most popular traditional bush teas of Senegal. The herbal beverage is traditionally used for weight loss, digestion, as a diuretic and mild antibiotic, and to relieve pain. The fresh leaves are used to treat malarial fever. Leaf extracts, the most biologically active plant tissue relative to stem, bark and roots, were screened for antioxidant capacity, measuring the removal of a radical by UV/VIS spectrophotometry, anti-inflammatory activity, measuring inducible nitric oxide synthase (iNOS) in RAW 264.7 macrophage cells, and glucose-lowering activity, measuring phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression in an H4IIE rat hepatoma cell line. Radical oxygen scavenging activity, or antioxidant capacity, was utilized for initially directing the fractionation; highlighted subfractions and isolated compounds were subsequently tested for anti-inflammatory and glucose-lowering activities. The ethyl acetate and n-butanol fractions of the crude leaf extract were fractionated leading to the isolation and identification of a number of polyphenolic ii compounds. -

WO 2017/050853 Al 30 March 2017 (30.03.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/050853 Al 30 March 2017 (30.03.2017) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12P 19/44 (2006.01) C12N 15/52 (2006.01) kind of national protection available): AE, AG, AL, AM, C12P 17/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP20 16/072474 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, 2 1 September 2016 (21 .09.201 6) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (25) Filing Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (26) Publication Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 62/222,919 24 September 2015 (24.09.2015) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: EVOLVA SA [CH/CH]; Duggingerstrasse 23, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 4153 Reinach (CH). -

EEE M W 24B 24A 27B 27A N Patent Application Publication Dec
US 2009031 1494A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0311494 A1 YAMASHTA et al. (43) Pub. Date: Dec. 17, 2009 (54) RELIEF PRINTING PLATE PRECURSOR FOR (30) Foreign Application Priority Data LASER ENGRAVING, RELIEF PRINTING PLATE, AND PROCESS FOR PRODUCING Jun. 17, 2008 (JP) ................................. 2008-157907 RELEF PRINTING PLATE Feb. 10, 2009 (JP) ................................. 2009-028816 (75) Inventors: Masako YAMASHITA, Publication Classification Shizuoka-ken (JP); Atsushi (51) Int. Cl. SUGASAKI, Shizuoka-ken (JP) B32B 3/00 (2006.01) Correspondence Address: GO3F 7/20 (2006.01) Moss & Burke, PLLC GO3F 7/004 (2006.01) 401 Holland Lane, Suite 407 Alexandria, VA 22314 (US) (52) U.S. Cl. .................... 428/195.1: 430/306: 430/286.1 (73) Assignee: FUJIFILM CORPORATION, (57) ABSTRACT Tokyo (JP) A relief printing plate precursor for laser engraving, including (21) Appl. No.: 12/476,260 a relief forming layer containing (A) a polymerizable com pound having an ethylenic unsaturated bond. (B) a binder (22) Filed: Jun. 2, 2009 polymer, and (C) a compound having deodorizing ability. 11 50 FA - 42 SUB SCANNING DIRECTION -10 - 228 7.s 55 21B EEE m w 24B 24A 27B 27A N Patent Application Publication Dec. 17, 2009 US 2009/0311494 A1 F.G. 1 FA SCANNING DIRECTION 7OA a. CSy ra & 5A - 27WSNS AD 23Ar S3EEASEE21 E-25sagaa EEEEEEEEEEEEEEEEEEEEEEEEE-22s awslighlights fskillsw. 21B 2 TTT "TT". US 2009/031 1494 A1 Dec. 17, 2009 RELEF PRINTING PLATE PRECURSORFOR mask to develop and remove an uncured area, and there is LASER ENGRAVING, RELIEF PRINTING room for improvement since development treatment is nec PLATE, AND PROCESS FOR PRODUCING essary. -

Raspberry Pomace - Composition, Properties and Application
View metadata, citation and similar papers at core.ac.uk brought to you by CORE European Journal ISSN 2449-8955 Review Article of Biological Research Raspberry pomace - composition, properties and application Agnieszka Joanna Brodowska Institute of General Food Chemistry, Faculty of Biotechnology and Food Sciences, Łódź University of Technology, Stefanowskiego 4/10, 90-924 Łódź, Poland * Corresponding author: Agnieszka Brodowska; E-mail: [email protected] Received: 19 February 2017; Revised submission: 24 March 2017; Accepted: 03 April 2017 Copyright: © The Author(s) 2017. European Journal of Biological Research © T.M.Karpi ński 2017. This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited. DOI : http://dx.doi.org/10.5281/zenodo.495190 ABSTRACT industry by-product, is considered as a potential food ingredient. Its pomace contains plenty of Raspberry pomace can be valorised due to its valuable components such as carbohydrates, nutritionally favourable effect on human health. proteins, fats, fibre, flavours, pectins, vitamins, It is an important source of polyphenols, ellagic similar to the composition of whole raspberries [2]. acid, ellagitannins, tocopherols, unsaturated fatty Moreover, raspberry pomace is rich in a large group acids, and dietary fibre. Thus, raspberry pomace of various phenolics especially ellagitannins, can be considered as a potential raw material to proanthocyanidins, anthocyanins, flavonols, and receive products rich in polyphenols or dietary phenolic acids (especially, ellagic acid) which are fibre, which can provide healthy properties to food also predominant in berries [3]. -

Sources of Crude Drug, Plant Families, Biogenesis of Phytochemicals
1 Sources of Crude Drug, Plant Families, Biogenesis of Phytochemicals SOURCES OF CRUDE DRUG Plant Oldest source of drugs. 25% of the drugs prescribed worldwide come from plants More than 200 drugs considered as basic and essential by the World Health Organisation (WHO) Significant number of synthetic drugs obtained from natural precursors. Example: Digoxin from Digitalis species, quinine and quinidine from Cinchona species, vincristrine and vinblastine from Catharanthus roseus, atropine from Atropa belladonna and morphine and codeine from Papaver somniferum. Animal Second largest source of crude drugs. Example: Honey from honeybee, beeswax from bees, cod liver oil from shark, bufalin from toad, animal pancreas is a source of Insulin, musk oil from musk, spermaceti wax from sperm whale, woolfat from sheep, carminic acid from colchineal, venoms from snake Mineral Highly purified form of naturally occurring mineral substances is used in medicine Example: Sulphur is a key ingredient in certain bacteriostatic drugs, shilajit is used as tonic, calamine is used as anti-itching agent Marine Major part of earth is covered with water bodies and hence bioactive compounds from marine flora and fauna (microorganisms, algae, fungi, invertebrates, and 1 Contd… 2 Pharmacognosy and Phytochemistry: A Companion Handbook vertebrates) have extensive past and present use in the treatment of many diseases Marine Serve as compounds of interest both in their natural form and as templates for synthetic modification. Several molecules isolated from various -

Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy
molecules Article Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy Valtteri Virtanen * , Susanna Räikkönen, Elina Puljula and Maarit Karonen Natural Chemistry Research Group, Department of Chemistry, University of Turku, FI-20014 Turku, Finland; [email protected] (S.R.); [email protected] (E.P.); maarit.karonen@utu.fi (M.K.) * Correspondence: vtjvir@utu.fi; Tel.: +358-29-450-3205 Abstract: Ellagitannins have antimicrobial activity, which might be related to their interactions with membrane lipids. We studied the interactions of 12 different ellagitannins and pentagalloylglucose with a lipid extract of Escherichia coli by high-resolution magic angle spinning NMR spectroscopy. The nuclear Overhauser effect was utilized to measure the cross relaxation rates between ellagitannin and lipid protons. The shifting of lipid signals in 1H NMR spectra of ellagitannin–lipid mixture due to ring current effect was also observed. The ellagitannins that showed interaction with lipids had clear structural similarities. All ellagitannins that had interactions with lipids had glucopy- ranose cores. In addition to the central polyol, the most important structural feature affecting the interaction seemed to be the structural flexibility of the ellagitannin. Even dimeric and trimeric ellagitannins could penetrate to the lipid bilayers if their structures were flexible with free galloyl and hexahydroxydiphenoyl groups. Keywords: E. coli; HR-MAS-NMR; interaction; lipid membrane; tannins; UPLC-DAD-MS Citation: Virtanen, V.; Räikkönen, S.; Puljula, E.; Karonen, M. 1. Introduction Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy. Tannins are a group of specialized plant metabolites, which, when included in the di- Molecules 2021, 26, 373. etary feed of ruminants, have been shown to induce many beneficial effects such as increas- https://doi.org/10.3390/ ing their effective amino acid absorption, lowering their methane production, and acting as molecules26020373 anthelmintics [1–6]. -

Phenolic Compounds in Cereal Grains and Their Health Benefits
and antioxidant activity are reported in the Phenolic Compounds in Cereal literature. Unfortunately, it is difficult to make comparisons of phenol and anti- Grains and Their Health Benefits oxidant activity levels in cereals since different methods have been used. The ➤ Whole grain cereals are a good source of phenolics. purpose of this article is to give an overview ➤ Black sorghums contain high levels of the unique 3-deoxyanthocyanidins. of phenolic compounds reported in whole ➤ Oats are the only source of avenanthramides. grain cereals and to compare their phenol and antioxidant activity levels. ➤ Among cereal grains, tannin sorghum and black rice contain the highest antioxidant activity in vitro. Phenolic Acids Phenolic acids are derivatives of benzoic and cinnamic acids (Fig. 1) and are present in all cereals (Table I). There are two Most of the literature on plant phenolics classes of phenolic acids: hydroxybenzoic L. DYKES AND L. W. ROONEY focuses mainly on those in fruits, acids and hydroxycinnamic acids. Hy- TEXAS A&M UNIVERSITY vegetables, wines, and teas (33,50,53,58, droxybenzoic acids include gallic, p- College Station, TX 74). However, many phenolic compounds hydroxybenzoic, vanillic, syringic, and in fruits and vegetables (i.e., phenolic acids protocatechuic acids. The hydroxycinna- esearch has shown that whole grain and flavonoids) are also reported in cereals. mic acids have a C6-C3 structure and Rconsumption helps lower the risk of The different species of grains have a great include coumaric, caffeic, ferulic, and cardiovascular disease, ischemic stroke, deal of diversity in their germplasm sinapic acids. The phenolic acids reported type II diabetes, metabolic syndrome, and resources, which can be exploited. -

ABSTRACT YUZUAK, SEYIT. Utilizing Metabolomics and Model Systems
ABSTRACT YUZUAK, SEYIT. Utilizing Metabolomics and Model Systems to Gain Insight into Precursors and Polymerization of Proanthocyanidins in Plants (Under the direction of Dr. De-Yu Xie). Proanthocyanidins (PAs) are oligomers or polymers of flavan-3-ols. In plants, PAs have multiple protective functions against biotic and abiotic stresses. PAs are also important nutraceuticals existing in common beverages and food products to benefit human health. To date, although the biosynthesis of PAs has been intensively studieed and fundamental progress has been made in over the past decades, many questions remain unanswered. For example, its direct precursors of extension units are unknown and their monomer structure diversity are also unclear. These questions about proanthocyanidins result from not having of model systems and effective technologies. Our laboratory has made significant progress in enhancing the understanding of these unknown and unclear points regarding proanthocyanidins. In my dissertation research reported here, I focus on two areas: testing muscadine as a crop system to develop metabolomics for studying precursor diversity and isolating enzymes from a red cell tobacco system to understand the precursors of the extension units of PA. First, I developed a metabolomics protocol to analyze anthocyanidins and anthocyanins in muscadine grape. This study demonstrated that muscadine berries can produce at least six anthocyanidins, revealing the diversity of pathway precursors for PAs. The Journal of Agriculture and Food Chemistry is reviewing this work. Second, I developed a metabolomics protocol of HPLC-qTOF-MS/MS to analyze flavan- 3-ols and dimeric PAs in muscadine grape. This study also demonstrated the high structure diversity of flavan-3-ols, particularly methylated flavan-3-ols. -

Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids
pharmaceutics Review Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids Giuliana Donadio 1,†, Francesca Mensitieri 2,†, Valentina Santoro 1, Valentina Parisi 1,3, Maria Laura Bellone 1,3, Nunziatina De Tommasi 1, Viviana Izzo 2 and Fabrizio Dal Piaz 2,* 1 Department of Pharmacy, University of Salerno, 84084 Fisciano, Italy; [email protected] (G.D.); [email protected] (V.S.); [email protected] (V.P.); [email protected] (M.L.B.); [email protected] (N.D.T.) 2 Department of Medicine and Surgery, University of Salerno, 84082 Baronissi, Italy; [email protected] (F.M.); [email protected] (V.I.) 3 PhD Program in Drug Discovery and Development, Department of Pharmacy, University of Salerno, 84084 Fisciano, Italy * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Flavonoids are among the most abundant natural bioactive compounds produced by plants. Many different activities have been reported for these secondary metabolites against numerous cells and systems. One of the most interesting is certainly the antimicrobial, which is stimulated through various molecular mechanisms. In fact, flavonoids are effective both in directly damaging the envelope of Gram-negative and Gram-positive bacteria but also by acting toward specific molecular targets essential for the survival of these microorganisms. The purpose of this paper is to present an overview of the most interesting results obtained in the research focused on the study of the Citation: Donadio, G.; Mensitieri, F.; interactions between flavonoids and bacterial proteins. Despite the great structural heterogeneity Santoro, V.; Parisi, V.; Bellone, M.L.; of these plant metabolites, it is interesting to observe that many flavonoids affect the same cellular De Tommasi, N.; Izzo, V.; Dal Piaz, F.