Simulation of Distillation Sequences for Acetone-Chloroform-Benzene Separation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards
A111D3 TTbS?? o z C/J NBS SPECIAL PUBLICATION 260-108 o ^EAU U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard Reference Materials: Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards QC 100 U57 R. Mavrodineanu and T. E. Gills 260-108 1987 m he National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal i s t0 strengthen and advance the nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research to assure international competitiveness and leadership of U.S. industry, science arid technology. NBS work involves development and transfer of measurements, standards and related science and technology, in support of continually improving U.S. productivity, product quality and reliability, innovation and underlying science and engineering. The Bureau's technical work is performed by the National Measurement Laboratory, the National Engineering Laboratory, the Institute for Computer Sciences and Technology, and the Institute for Materials Science and Engineering. The National Measurement Laboratory Provides the national system of physical and chemical measurement; • Basic Standards 2 coordinates the system with measurement systems of other nations and • Radiation Research furnishes essential services leading to accurate and uniform physical and • Chemical Physics chemical measurement throughout the Nation's scientific community, • Analytical Chemistry industry, and commerce; provides advisory and research services to other Government agencies; conducts physical and chemical research; develops, produces, and distributes Standard Reference Materials; provides calibration services; and manages the National Standard Reference Data System. -

BENZENE AS a LARVICIDE for SCREW WORMS1 the Larval Stage
BENZENE AS A LARVICIDE FOR SCREW WORMS1 By D. C. PARMAN Assistant Entomologist, Investigations of Insects Affecting the Health of Animals, Bureau of Entomology, United States Department of Agriculture INTRODUCTION The larval stage of CocMiomyia macellaria Fab., generally known amonff stock raisers in the Southwest as the screw worm, causes con- siderable loss to the livestock industry, estimated as high as $5,000,000 in some years. It has been apparent that the larvicides used to kill the worms are either toxic to the animal or at least in most cases detrimental to the healing of the wounds. This toxicity was at first attributed to the screw worm, but as many cases were ob- served where the animal was practically consumed by the larvae and still lived until the loss of olood or injury to some vital organ brought de^th, it was surmised that the treatments with larvicides were the cause of many deaths. During the summer of 1916 syste- matic work was begun to find a more efficient larvicide than the phenols and chloroform which were generally used. At first an attempt was made to add something to these larvicides to counteract the toxic properties. As this was not successful it was deemed best to look for a chemical that might be used with more satis- factory results. Several chemical groups were studied for possible larvicides. EXPERIMENTAL PROCEDURE All available chemicals with possible larvicidal value were selected for laboratory tests to determine whether they would kill the larvae of the screw-worm fly. The first tests were made by pouring the chemical on a number of larvae in a tube, or dusting on just enough to cover the larvae. -

Sds – Safety Data Sheet
Effective Date: 02/05/16 Replaces Revision: 01/01/13, 02/26/09 NON-EMERGENCY TELEPHONE 24-HOUR CHEMTREC EMERGENCY TELEPHONE 610-866-4225 800-424-9300 SDS – SAFETY DATA SHEET 1. Identification Product Identifier: ACETONE / ISOPROPYL ALCOHOL BLEND Synonyms: None Chemical Formula: Not Applicable to mixtures Recommended Use of the Chemical and Restrictions On Use: Laboratory Reagent Manufacturer / Supplier: Puritan Products; 2290 Avenue A, Bethlehem, PA 18017 Phone: 610-866-4225 Emergency Phone Number: 24-Hour Chemtrec Emergency Telephone 800-424-9300 2. Hazard(s) Identification Classification of the Substance or Mixture: Flammable liquids (Category 2) Skin irritation (Category 3) Eye irritation (Category 2A) Specific target organ toxicity - single exposure (Category 3) Risk Phrases: R11: Highly flammable. R36: Irritating to eyes. R66: Repeated exposure may cause skin dryness or cracking. R67: Vapors may cause drowsiness and dizziness. Label Elements: Trade Name: ACETONE / ISOPROPYL ALCOHOL BLEND Signal Word: Danger Hazard Statements: H225: Highly flammable liquid and vapor. H316: Causes mild skin irritation. H319: Causes serious eye irritation. H336: May cause drowsiness or dizziness. ACETONE / ISOPROPYL ALCOHOL BLEND Page 1 of 6 Precautionary Statements: P210: Keep away from heat/sparks/open flames/hot surfaces. No smoking. P261: Avoid breathing dust / fume / gas / mist / vapors / spray. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. 3. Composition / Information on Ingredients CAS Number: Not Applicable to mixtures Molecular Weight: Not Applicable to mixtures Ingredient CAS Number EC Number Percent Hazardous Chemical Characterization Acetone 67 - 64 - 1 200-662-2 70 - 90% Yes Substance Isopropyl Alcohol 67 - 63 - 0 200-661-7 10 - 30% Yes Substance 4. -

SAFETY DATA SHEET Acetone
SAFETY DATA SHEET Acetone Revision date: 12.05.15 SDS/004/8 Page 1 of 6 1. Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Acetone EC No. 200-662-2 1.2. Relevant identified uses of the substance or mixture and uses advised against Household Solvent 1.3. Details of the supplier of the safety data sheet Thornton & Ross Ltd, Linthwaite, Huddersfield, HD7 5QH Tel: 01484 842217 Fax: 01484 847301 Email: [email protected] 1.4 Emergency telephone number: Out of normal working hours: +44 870 8510207 2. Hazards identification 2.1. Classification of the substance or mixture According to Regulation EC 1272/2008 classified as Flammable Liquid Category 2, Eye Irritant Category 2, Specific Target Organ Toxicity Single Exposure Category 3. 2.2. Label element GHS Pictogram Signal Word Hazard Class Danger Flammable Liquids, Category 2 Eye Irritation, Category 2 Specific Target Organ Toxicity-Single Exposure, Category 3 Hazard Statements Precautionary statements H225: Highly flammable liquid and vapour. P210: Keep away from heat/sparks/open flames/hot surfaces – No smoking. H319: Causes serious eye irritation P305+351+338: IF IN EYES: Rinse cautiously with H336: May cause drowsiness or dizziness water for several minutes. Remove contact lenses if present and easy to do – continue rinsing. P337+313: Get medical advice/attention. P403: Store in a well ventilated place. Supplemental Hazard Information (EU) EUH066: Repeated exposure may cause skin dryness or cracking 2.3. Other hazards N/A SAFETY DATA SHEET Acetone Revision date: 12.05.15 SDS/004/8 Page 2 of 6 3. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Formation of Nitrous Oxide
1461 No. 5069 DECEMBER 24. 1966 NATURE E-+ CCI. --->- ECC1 3 + Cl- Formation of Nitrous Oxide THE mechanism of formation of nitrous oxide from am The tightly bound .CCI3 group will not be removable in monium nitrate is attributable to the recognized thermal the normal biochemical reaction and the enzyme molecule dissociation of ammonium salts followed in this instance will be inactivated. by nitration of ammonia and decomposition of the ensuing This process of enzyme inactivation could, in principle, nitramide. The mechanism of this latter stage has been account for the toxicity of carbon tetrachloride and studied by various workers, notably by Marlies and La similar compounds and also of many insecticides. The Merl. Further, the decomposition of methyl nitramido postulated mechanism would also explain the observation to tho oxide and methyl alcohol is on record 2. by Butlerl3 that carbon tetrachloride is reduced in vivo As the nitration process, for example, of benzene, is to chloroform. Thus a proportion of .CCls radicals may commonly accompanied by some oxidation, the possibility react with sites other than the enzyme and abstract, of its OCClllTcnce in the preparation of nitrous oxide with for example, a hydrogen atom from a hydroxyl group or formation of nitrogen peroxide is not surprising and from a sulphydryl group. The latter exchange does technically well recognized. It has also recently been the appear to be possible from energy considerations. subject of public concern. D(RS-H) = 89 kcalJmole (ref. 14), .J. KENNER D(CI3G-H) = 91·4 kcalJmole (ref. 9) 41 Burlington Road, I have found that the activation energy of the electron Withington, capture reaction in chloroform vapour Manchester 20. -

Toxicological Profile for Acetone Draft for Public Comment
ACETONE 1 Toxicological Profile for Acetone Draft for Public Comment July 2021 ***DRAFT FOR PUBLIC COMMENT*** ACETONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** ACETONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -

Annexes To: CPMP/ICH/283/95 Impurities: Guideline for Residual Solvents & CVMP/VICH/502/99 Guideline on Impurities
20 February 2013 CPMP/QWP/450/03 -Rev.1, EMEA/CVMP/511/03 -Rev.1 Committee for medicinal products for human use (CHMP) Committee for medicinal products for veterinary use (CVMP) Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Annex I: specifications for class 1 and class 2 residual solvents in active substances Annex II: residues of solvents used in the manufacture of finished products Discussion at Quality Working Party January 2003 to June 2004 Adoption by CVMP July 2004 Adoption by CHMP July 2004 Date for coming into operation January 2005 Rev. 01 Adoption by Quality Working Party 22 November 2012 Rev. 01 Adoption by CVMP 7 February 2013 Rev. 01 Adoption by CHMP 11 February 2013 Rev. 01 Date for coming into operation 1 March 2013 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Introduction The two (V)ICH residual solvents guidelines, ICH Q3C Impurities: Guideline for residual solvents (CPMP/ICH/283/95) and VICH GL18 Guideline on impurities: residual solvents in new veterinary medicinal products, active substances and excipients (CVMP/VICH/502/99), have been in operation for several years, since March 1998 and June 2001 respectively. -

Acetone - Toxfaqs™
Acetone - ToxFAQs™ What is acetone? Acetone is a (manmade) chemical that can also be found in the environment. It is a colorless liquid with a distinct smell and taste. It evaporates easily in air, is flammable, and dissolves in water. Acetone is used by humans to dissolve other substances and to produce plastics, paints and coatings, cleaning products, and personal care products. Other manmade sources of acetone are vehicle exhaust, tobacco smoke, and landfills. Acetone is also released naturally by plants, trees, insects, microbes (germs), volcanic erruptions, and forest fires, and can be found naturally in many fruits and vegetables. Low levels of acetone are produced naturally by the human body, and some health conditions can cause these levels to increase. What happens to acetone in the environment? • Most acetone in the environment exists as vapor in the air, and it can travel long distances this way. • About half of the total acetone in air is broken down by sunlight or other chemicals within 22 days. • Acetone moves from the air into water and soil by rain and snow, and moves quickly from water and soil back into the air. It does not bind to soil or build up in animals. • Acetone can enter surface water as manufacturing waste and seep into groundwater from landfills. • Acetone is broken down by microbes or chemicals in water and soil. How can I be exposed to acetone? A strong scent of acetone • Low levels of acetone are in the air, so most people are exposed and irritation in your eyes, to very small amounts through breathing, but these are rarely at nose, and throat are levels that are harmful to your health. -
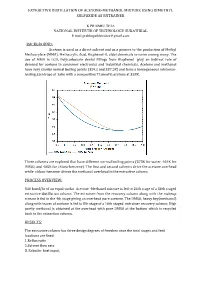
Extractive Distillation of Acetone-Methanol Mixture Using Dimethyl Sulfoxide As Entrainer
EXTRACTIVE DISTILLATION OF ACETONE-METHANOL MIXTURE USING DIMETHYL SULFOXIDE AS ENTRAINER K PRABHU TEJA NATIONAL INSTITUTE OF TECHNOLOGY SURATHKAL E mail:[email protected] BACKGROUND: Acetone is used as a direct solvent and as a pioneer to the production of Methyl Methacrylate (MMA), Methacrylic Acid, Bisphenol-A, aldol chemicals to name among many. The use of MMA in LCD, Polycarbonate dental fillings from Bisphenol play an indirect role of demand for acetone in consumer electronics and industrial chemicals. Acetone and methanol have very similar normal boiling points (329.2 and 337.5K) and form a homogeneous minimum- boiling azeotrope at 1atm with a composition77.6mol% acetone at 328K. Three solvents are explored that have different normal boiling points (373K for water, 464K for DMSO, and 405K for chloro-benzene). The rst and second solvents drive the acetone overhead while chloro-benzene drives the methanol overhead in the extractive column. fi PROCESS OVERVIEW: 540 kmol/hr of an equal-molar Acetone -Methanol mixture is fed to 24th stage of a 36th staged extractive distillation column. The entrainer from the recovery column along with the makeup stream is fed to the 4th stage giving an overhead pure acetone. The DMSO, heavy key(methanol) along with traces of acetone is fed to 8th stage of a 16th staged entrainer recovery column. High purity methanol is obtained at the overhead with pure DMSO at the bottom which is recycled back to the extraction column. RESULTS: The extractive column has three design degrees of freedom once the total stages and feed locations are fixed: 3..Reflux Reboiler ratio heat input. -

Optimization Study on the Azeotropic Distillation Process for Isopropyl Alcohol Dehydration
Korean J. Chem. Eng., 23(1), 1-7 (2006) Optimization study on the azeotropic distillation process for isopropyl alcohol dehydration Jungho Cho and Jong-Ki Jeon*,† Department of Chemical Engineering, Dong Yang University, 1, Kyochon-dong, Poongki-eup, Youngju, Gyeongbuk 750-711, Korea *Department of Chemical Engineering, Kongju National University, 182, Shinkwan-dong, Gongju, Chungnam 314-701, Korea (Received 9 August 2005 • accepted 26 November 2005) Abstract−Modeling and optimization work was performed using benzene as an entrainer to obtain a nearly pure an- hydrous isopropyl alcohol product from dilute aqueous IPA mixture through an azeotropic distillation process. NRTL liquid activity coefficient model and PRO/II with PROVISION 6.01, a commercial process simulator, were used to simulate the overall azeotropic distillation process. We determined the total reboiler heat duties as an objective function and the concentration of IPA at concentrator top as a manipulated variable. As a result, 38.7 mole percent of IPA at concentrator top gave the optimum value that minimized the total reboiler heat duties of the three distillation columns. Key words: Azeotropic Distillation, Entrainer, NRTL Liquid Activity Coefficient Model, Simulation, Optimization INTRODUCTION using special distillation. One is to utilize an azeotropic distillation process using benzene or cyclohexane as an entrainer [Font et al., An anhydrous isopropyl alcohol (IPA) product with purity over 2003; Tao et al., 2003; Al-Amer, 2000; Fele et al., 2000] and the 99.9% by weight has been widely used as a raw material of paint other is an extractive distillation process using ethylene glycol as a or ink products and as a solvent in electronic and medicine indus- solvent [Ligero and Ravagnani, 2003; Pinto et al., 2000].