(Hemiptera: Pseudococcidae) in Costa Rica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abiotic and Biotic Pest Refuges Hamper Biological Control of Mealybugs in California Vineyards K.M
____________________________________ Abiotic and biotic pest refuges in California vineyards 389 ABIOTIC AND BIOTIC PEST REFUGES HAMPER BIOLOGICAL CONTROL OF MEALYBUGS IN CALIFORNIA VINEYARDS K.M. Daane,1 R. Malakar-Kuenen,1 M. Guillén,2 W.J. Bentley3, M. Bianchi,4 and D. González,2 1 Division of Insect Biology, University of California, Berkeley, California, U.S.A. 2 Department of Entomology, University of California, Riverside, California, U.S.A. 3 University of California Statewide IPM Program, Kearney Agricultural Center, Parlier, California, U.S.A. 4 University of California Cooperative Extension, San Luis Obispo, California, U.S.A. INTRODUCTION Four mealybug species cause economic damage in California vineyards. These are the grape mealy- bug, Pseudococcus maritimus (Ehrhorn); obscure mealybug, Pseudococcus viburni (Signoret); longtailed mealybug, Pseudococcus longispinus (Targioni-Tozzeti); and vine mealybug, Planococcus ficus (Signoret) (Godfrey et al., 2002). The grape, obscure, and longtailed mealybugs belong to the Pseudococcus maritimus-malacearum complex–a taxonomically close group of mealybugs (Wilkey and McKenzie, 1961). However, while the origins of the grape and longtailed mealybugs are believed to be in North America, the ancestral lines of the obscure mealybug are unclear. Regardless, these three species have been known as pests in North America for nearly 100 years. The vine mealybug, in contrast, was first identified in California in the Coachella Valley in the early 1990s (Gill, 1994). It has since spread into California’s San Joaquin Valley and central coast regions, with new infestations reported each year. The four species are similar in appearance; however, mealybugs in the P. maritimus- malacearum complex have longer caudal filaments than vine mealybug (Godfrey et al., 2002). -

Review of Ecologically-Based Pest Management in California Vineyards
insects Review Review of Ecologically-Based Pest Management in California Vineyards Houston Wilson 1,* and Kent M. Daane 2 ID 1 Department of Entomology, University of California, Riverside, Riverside, CA 92521, USA 2 Department Environmental Science, Policy and Management, University of California, Berkeley, Berkeley, CA 94720-3114, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-559-646-6519 Academic Editors: Alberto Pozzebon, Carlo Duso, Gregory M. Loeb and Geoff M. Gurr Received: 28 July 2017; Accepted: 6 October 2017; Published: 11 October 2017 Abstract: Grape growers in California utilize a variety of biological, cultural, and chemical approaches for the management of insect and mite pests in vineyards. This combination of strategies falls within the integrated pest management (IPM) framework, which is considered to be the dominant pest management paradigm in vineyards. While the adoption of IPM has led to notable and significant reductions in the environmental impacts of grape production, some growers are becoming interested in the use of an explicitly non-pesticide approach to pest management that is broadly referred to as ecologically-based pest management (EBPM). Essentially a subset of IPM strategies, EBPM places strong emphasis on practices such as habitat management, natural enemy augmentation and conservation, and animal integration. Here, we summarize the range and known efficacy of EBPM practices utilized in California vineyards, followed by a discussion of research needs and future policy directions. EBPM should in no way be seen in opposition, or as an alternative to the IPM framework. Rather, the further development of more reliable EBPM practices could contribute to the robustness of IPM strategies available to grape growers. -

Evaluation of RNA Interference for Control of the Grape Mealybug Pseudococcus Maritimus (Hemiptera: Pseudococcidae)
insects Article Evaluation of RNA Interference for Control of the Grape Mealybug Pseudococcus maritimus (Hemiptera: Pseudococcidae) Arinder K. Arora 1, Noah Clark 1, Karen S. Wentworth 2, Stephen Hesler 2, Marc Fuchs 3 , Greg Loeb 2 and Angela E. Douglas 1,4,* 1 Department of Entomology, Cornell University, Ithaca, NY 14850, USA; [email protected] (A.K.A.); [email protected] (N.C.) 2 Department of Entomology, Cornell University, Geneva, NY 14456, USA; [email protected] (K.S.W.); [email protected] (S.H.); [email protected] (G.L.) 3 School of Integrative Plant Science, Cornell University, Geneva, NY 14456, USA; [email protected] 4 Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853, USA * Correspondence: [email protected] Received: 19 September 2020; Accepted: 26 October 2020; Published: 28 October 2020 Simple Summary: RNA interference (RNAi) is a defense mechanism that protects insects from viruses by targeting and degrading RNA. This feature has been exploited to reduce the expression of endogenous RNA for determining functions of various genes and for killing insect pests by targeting genes that are vital for insect survival. When dsRNA matching perfectly to the target RNA is administered, the RNAi machinery dices the dsRNA into ~21 bp fragments (known as siRNAs) and one strand of siRNA is employed by the RNAi machinery to target and degrade the target RNA. In this study we used a cocktail of dsRNAs targeting grape mealybug’s aquaporin and sucrase genes to kill the insect. Aquaporins and sucrases are important genes enabling these insects to maintain water relations indispensable for survival and digest complex sugars in the diet of plant sap-feeding insects, including mealybugs. -

An Investigation Into the Integrated Pest Management of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Stellenbosch University SUNScholar Repository An investigation into the integrated pest management of the obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), in pome fruit orchards in the Western Cape Province, South Africa. Pride Mudavanhu Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Agriculture (Entomology), in the Faculty of AgriSciences. at the University of Stellenbosch Supervisor: Dr Pia Addison Department of Conservation Ecology and Entomology Faculty of AgriSciences University of Stellenbosch South Africa December 2009 DECLARATION By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the owner of the copyright thereof (unless to the extent explicitly otherwise stated) and that I have not previously in its entirety or in part submitted it for obtaining any qualification. December 2009 Copyright © 2009 Stellenbosch University All rights reserved i ABSTRACT Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae) (obscure mealybug), is a common and serious pest of apples and pears in South Africa. Consumer and regulatory pressure to produce commodities under sustainable and ecologically compatible conditions has rendered chemical control options increasingly limited. Information on the seasonal occurrence of pests is but one of the vital components of an effective and sustainable integrated pest management system needed for planning the initiation of monitoring and determining when damage can be expected. It is also important to identify which orchards are at risk of developing mealybug infestations while development of effective and early monitoring tools for mealybug populations will help growers in making decisions with regards to pest management and crop suitability for various markets. -

Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas
Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas Neal L. Evenhuis, Lucius G. Eldredge, Keith T. Arakaki, Darcy Oishi, Janis N. Garcia & William P. Haines Pacific Biological Survey, Bishop Museum, Honolulu, Hawaii 96817 Final Report November 2010 Prepared for: U.S. Fish and Wildlife Service, Pacific Islands Fish & Wildlife Office Honolulu, Hawaii Evenhuis et al. — Pagan Island Arthropod Survey 2 BISHOP MUSEUM The State Museum of Natural and Cultural History 1525 Bernice Street Honolulu, Hawai’i 96817–2704, USA Copyright© 2010 Bishop Museum All Rights Reserved Printed in the United States of America Contribution No. 2010-015 to the Pacific Biological Survey Evenhuis et al. — Pagan Island Arthropod Survey 3 TABLE OF CONTENTS Executive Summary ......................................................................................................... 5 Background ..................................................................................................................... 7 General History .............................................................................................................. 10 Previous Expeditions to Pagan Surveying Terrestrial Arthropods ................................ 12 Current Survey and List of Collecting Sites .................................................................. 18 Sampling Methods ......................................................................................................... 25 Survey Results .............................................................................................................. -

Zootaxa, Hemiptera, Pseudococcidae
Zootaxa 964: 1–8 (2005) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 964 Copyright © 2005 Magnolia Press ISSN 1175-5334 (online edition) A new pest of tomato and other records of mealybugs (Hemiptera: Pseudococcidae) from Espírito Santo, Brazil MARK P. CULIK1 & PENNY J. GULLAN2 1Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural — INCAPER, Rua Afonso Sarlo 160, CEP 29052-010, Vitória, Espírito Santo, Brasil, e-mail: [email protected]; 2Department of Entomology, University of California, One Shields Avenue, Davis, CA 95616-8584, USA, e-mail: [email protected]. Abstract Three mealybug (Hemiptera: Pseudococcidae) plant pest species: Dysmicoccus boninsis (Kuwana), Phenacoccus solenopsis Tinsley, and Pseudococcus viburni (Signoret), are recorded for the first time in the state of Espírito Santo, Brazil. This is the first record of Phenacoccus solenopsis in Bra- zil, where it was found infesting tomato plants. The species Antonina graminis (Maskell), a com- mon pest of Bermuda grass, and Dysmicoccus brevipes (Cockerell), a major pest of pineapple, also were encountered. Key words: Antonina graminis, Dysmicoccus boninsis, Dysmicoccus brevipes, Phenacoccus sole- nopsis, Pseudococcus viburni, Solanum lycopersicum Uma nova praga do tomateiro e outros registros de cochonilhas-farinhentas (Hemi- ptera: Pseudococcidae) no Espírito Santo, Brasil Resumo: São registradas pela primeira vez a ocorrência das cochonilhas-farinhentas (Hemiptera: Pseudococcidae) Dysmicoccus boninsis (Kuwana), Phenacoccus solenopsis Tinsley, e Pseudococ- cus viburni (Signoret) no estado do Espírito Santo. Destaca-se que o registro de Phenacoccus sole- nopsis, encontrada no tomateiro, é o primeiro no Brasil. Também foram encontradas em capim Bermuda a espécie Antonina graminis (Maskell) e em abacaxi e abóbora a espécie Dysmicoccus brevipes (Cockerell), ambas já relatadas no estado do Espírito Santo. -

Blank Document
Application to release the microhymenopteran parasitoid Tachardiaephagus somervillei for the control of the invasive scale insect Tachardina aurantiaca on Christmas Island, Indian Ocean Prepared by Peter T. Green, Dennis J. O’Dowd and Gabor Neumann (La Trobe University, Kingsbury Drive, Bundoora 3086) on behalf the Director of National Parks. Submitted by The Director of National Parks, for assessment by the Australian Government Department of Agriculture 1 December 2014 Contents Executive Summary ………………………………………………………………………………………………………………………………..iii Preamble ………………………………………………………………………………………………………………………………………………. vi Acknowledgments ……………………………………………………………………………………………………………………………… viii 1. Information on the target species, the yellow lac scale Tachardina aurantiaca ……………………………. 1 1.1 Taxonomy ………………………………………………………………………………………………………………………….. 1 1.2 Description ………………………………………………………………………………………………………………………… 1 1.3 Distribution ……………………………………………………………………………………………………………………….. 1 1.4 Australian Range ………………………………………………………………………………………………………………… 2 1.5 Ecology ………………………………………………………………………………………………………………………………. 2 1.6 Impacts ……………………………………………………………………………………………………………………………. 3 1.7 Information on all other relevant Commonwealth, State and Territory legislative controls of the target species …………………………………………………………………………… 7 1.8 When the target was approved for biological control ………………………………………………………. 7 1.9 History of biological control ……………………………………………………………………………………………… 7 2. Information on the potential agent Tachardiaephagus somervillei ……………………………………………. -

FICHA TÉCNICA Dysmicoccus Neobrevipes
FICHA TÉCNICA Dysmicoccus neobrevipes (Bearsley, 1959) (Hemiptera: Pseudococcidae) Cochinilla gris Créditos: Joshi, s/a, Rung, 2018 . CONTENIDO IDENTIDAD DE LA PLAGA ................................ ................................ ................................ ................................ ................................ ........ 1 Nombre científico ..................................................................................................................................................................................... 1 Sinonimias ........................................................................................................................................................................................................... 1 Clasificación taxonómica ...................................................................................................................................................................... 1 Nombre común ............................................................................................................................................................................................... 1 ESTATUS FITOSANITARIO EN MÉXICO ............................................................................................................................................. 1 IMPORTANCIA ECONÓMICA DE LA PLAGA ................................................................................................................................. 2 DISTRIBUCIÓN MUNDIAL ....................................................................................................................................................................... -

Transmission of Grapevine Leafroll-Associated Virus 3 (Glrav-3): Acquisition, Inoculation and Retention by the Mealybugs Planococcus Ficus and Pseudococcus Longispinus (Hemiptera
Transmission of Grapevine Leafroll-associated Virus 3 (GLRaV-3): Acquisition, Inoculation and Retention by the Mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae) K. Krüger1,*, D.L. Saccaggi1,3, M. van der Merwe2, G.G.F. Kasdorf2 (1) Department of Zoology & Entomology, University of Pretoria, Private Bag X20, Pretoria, 0028, South Africa (2) ARC-Plant Protection Research Institute, Private Bag X134, Pretoria, 0001, South Africa (3) Current address: Plant Health Diagnostic Services, Department of Agriculture, Forestry and Fisheries, Private Bag X5015, Stellenbosch, 7599, South Africa Submitted for publication: December 2014 Accepted for publication: March 2015 Key words: Ampelovirus, Closteroviridae, Coccoidea, grapevine leafroll disease, Vitis vinifera The vine mealybug, Planococcus ficus (Signoret), and the longtailed mealybug, Pseudococcus longispinus (Targioni Tozzetti), are vectors of grapevine leafroll-associated virus 3 (GRLaV-3), one of the most abundant viruses associated with grapevine leafroll disease. To elucidate the transmission biology in South Africa, acquisition access periods (AAPs), inoculation access periods (IAPs) and the retention of the virus in starving and feeding first- to second instar nymphs were determined. The rootstock hybrid LN33 served as virus source and grapevines (Vitis vinifera L., cv. Cabernet franc) served as recipient plants. An AAP of 15 min or an IAP of 15 min was sufficient forPl. ficus to acquire or transmit GLRaV-3, respectively. Nymphs of Pl. ficus retained the virus for at least eight days when feeding on a non-virus host and grapevine, and for at least two days when starving, and were then capable of transmitting it successfully to healthy grapevine plants. Nymphs of Ps. longispinus transmitted the virus after an AAP of 30 min and an IAP of 1 h. -

Little Cherry Virus 1 & 2
WASHINGTON STATE UNIVERSITY EXTENSION Little Cherry Virus 1 & 2 Written by: Scott Harper, WSU Plant Pathology; Andrea Bixby Brosi, Betsy Beers, WSU Entomology; Tianna DuPont, WSU Extension. May 2019. Little Cherry Disease (LCD) is a critical concern to sweet cherry producers in the state of Washington. This disease is caused by infection by Little cherry virus 1 (LChV1), Little cherry virus 2 (LChV2) or the X-Disease Phytoplasma (Candidatus Phytoplasma pruni), and produces small, undersized cherries, with poor color development and flavor. Background Little Cherry Disease is not a new problem in the Pacific Figure 2 Little Cherry Virus 2 produces cherries of small size and poor Northwest. The first major epidemic occurred in the Kootenay color and flavor. Photo credit Andrea Bixby Brosi, WSU. valley in Canada in 1938, and over the following 40 years Little cherry virus (both -1 and-2) infection of sweet cherry devastated the Canadian cherry industry so much that by 1979 cultivars results in small fruit with reduced sugar content that the last packing line in the Kootenay valley closed, and the may taste bland or insipid. Severity of the disease differs Canadian industry has spent the last 40 years rebuilding. between cultivars, with Lambert and Bing being highly In Washington State the last major epidemic occurred in the late susceptible, whereas Van and Sam are less so and fruit may 1940s and into the 1950s, resulting in the removal of significant reach marketable size although flavor is still affected. On some acreage. But, with the passage of time and changes in cultivars such as Lambert, the fruit may also have a noticeable management practices, the disease has become prevalent in pointed tip. -

Standards Committee
REPORT (REVISED 2016-01-06, SECTION 11.3) Standards Rome, Italy Committee 16-20 November 2015 November, 2015 Food and Agriculture Organization of the United Nations SC November 2015 Report Contents 1. Opening of the meeting .............................................................................................................. 4 1.1 Welcome by the IPPC Secretariat ............................................................................... 4 1.2 Election of the Rapporteur .......................................................................................... 5 1.3 Adoption of the Agenda Chairperson .......................................................................... 5 2. Administrative Matters ............................................................................................................... 5 3. Updates ....................................................................................................................................... 5 3.1 Items arising from governance bodies......................................................................... 5 3.2 Briefings from IPPC Secretariat .................................................................................. 6 4. Draft ISPMs for recommendation to CPM (from Substantial concerns commenting period) .... 9 4.1 2014 Amendments to ISPM 5 (Glossary of phytosanitary terms) (1994-001) ........... 9 4.2 Draft ISPM on Determination of host status of fruit to fruit fly (Tephritidae) (2006- 031), Priority 1 ......................................................................................................... -
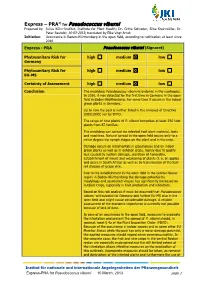
Express PRA Pseudococcus Viburni
1) Express – PRA for Pseudococcus viburni Prepared by: Julius Kühn-Institut, Institute for Plant Health; Dr. Gritta Schrader, Silke Steinmöller, Dr. Peter Baufeld; 10-03-2013; translated by Elke Vogt-Arndt Initiation: Occurrence in Baden-Württemberg in the open field, according to notification at least since 2010 Express - PRA Pseudococcus viburni (Signoret) Phytosanitary Risk for high medium low Germany Phytosanitary Risk for high medium low EU-MS Certainty of Assessment high medium low Conclusion The mealybug Pseudococcus viburni is endemic in the neotropics. In 2010, it was detected for the first time in Germany in the open field in Baden-Württemberg. For some time it occurs in the indoor green plants in Germany. Up to now the pest is neither listed in the annexes of Directive 2000/29/EC nor by EPPO. The range of host plants of P. viburni comprises at least 296 host plants from 87 families. The mealybug can spread via infested host plant material, tools and machines. Natural spread in the open field occurs only to a minor degree via nymph stages on the plant and in the crop. Damage occurs on ornamentals in glasshouses and on indoor green plants as well as in outdoor crops, mainly due to quality loss caused by suction damage, secretion of honeydew, establishment of mould and weakening of plants (f. e. on apples and pears in South Africa) as well as by transmission of the leaf- roll disease of grape vine. Due to the establishment in the open field in the central Neckar region in Baden-Württemberg the damage potential by mealybugs and associated viruses has significantly increased on outdoor crops, especially in fruit production and viticulture.