Curriculum Faculty of Sciences the Women
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Annual Report
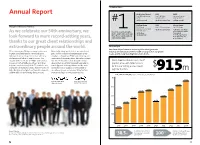
Top Ranking Report Annual Report Architectural Record ENR VMSD Top 300 Architecture Top 150 Global Top Retail Design Firms: Design Firms: Firms of 2014: # #1 Firm Overall #1 Architecture Firm #1 Firm Overall Building Design ENR Interior Design Message from the Board of Directors 2014 World Top 500 Design Firms: Top 100 Giants: Architecture 100 Most #1 Architecture Firm #1 Architecture Firm Admired Firms: Gensler is1 a leader among the #1 in Corporate Office As we celebrate our 50th anniversary, we world’s architecture and design #1 US Firm #1 in Retail #4 Global Firm #1 in Transportation firms. Here’s how we ranked in #1 in Government look forward to more record-setting years, our industry in 2014. #1 in Cultural thanks to our great client relationships and extraordinary people around the world. Financial Report Our financial performance and recognition throughout the We’re entering our 50th year stronger than ever. Financially strong and debt-free, we contributed industry are indications of the breadth of our practice, our global In 2014, our global growth continued apace $38.5 million in deferred compensation to our reach, and the long-standing trust of our clients. with our clients as they entrusted us with new employees through our ESOP, profit-sharing, and challenges and led us to new locations. Our international retirement plans. We made strategic expanded Gensler team of 4,700+ professionals investments in our research and professional We’ve broadened our services to 27 now work from 46 different offices. With their development programs, along with upgrades to practice areas, with total revenues help, we completed projects in 72 countries and our design-and-delivery platform and the tools for the year setting a new record $ increased our revenues to $915 million—a record and technology to support it. -

Classnotes: Summer 2016
motorhome (a 2003 Winnebago Brave, which degree in May 2015. I can’t imagine why celebrating, Maxine is busy knitting for each we lived in full time for a year or so, traveling) it took so long. My wife, Elaine (Norris) new addition to the extended family. and will use it this summer for an extended C’60, P’93, and I visited him a couple of Bob and Mary (Peck) C’62 Davidson also trip east to see family and friends. Younger times while he was at Wabash. It was clear had to skip the reunion as their grandson, daughter Linda’s husband has a job teaching that he had become a much-loved school Andrew, was graduating from high school at the University of Surrey Law School, so they institution—much as he was at Drew— in June. Their oldest son, John, will retire will be moving from Harvard Law School and had a remarkable rapport with his from the Marin County Fire Department to England soon. Elder daughter Jennifer students. John lived in a small house on after 30 years as a firefighter and EMT. He’s (mother of the grandkids) is a histological the edge of campus, and we still remember a licensed alligator hunter in Florida and technician making pathology slides for Mohs one Saturday during Homecoming Week, recently caught 8- and 9-foot gators. skin cancer surgery. I continue with my when wave after wave of students rang John and Mary-Ann (Kennerly) Clinton Dickens study group, as we traverse all of his doorbell to introduce their girlfriends, have been in Lincoln, Nebraska, for four Dickens fiction. -

Web Application Architecture: Principles, Protocols and Practices
Web Application Architecture Principles, protocols and practices Leon Shklar Richard Rosen Dow Jones and Company Web Application Architecture Web Application Architecture Principles, protocols and practices Leon Shklar Richard Rosen Dow Jones and Company Copyright 2003 by John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England Telephone (+44) 1243 779777 Email (for orders and customer service enquiries): [email protected] Visit our Home Page on www.wileyeurope.com or www.wiley.com All Rights Reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except under the terms of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London W1T 4LP, UK, without the permission in writing of the Publisher with the exception of any material supplied specifically for the purpose of being entered and executed on a computer system for exclusive use by the purchase of the publication. Requests to the Publisher should be addressed to the Permissions Department, John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England, or emailed to [email protected], or faxed to (+44) 1243 770620. This publication is designed to provide accurate and authoritative information in regard to the subject matter covered. It is sold on the understanding that the Publisher is not engaged in rendering professional services. If professional advice or other expert assistance is required, the services of a competent professional should be sought. -

Classnotes: Fall 2019 Written by Kmerusi
DREW CLASSNOTES The College of Liberal Arts Ronald Vander Schaaf C’56, T’59 One of the most fit men in our class has September I went to Sloss Furnace, a defunct 56 [email protected] got to be Roy Haynes. He entered a 5K race steel mill, to see the dedication of a plaque Prunella (Read) Williams, Harold in Wooster, Ohio, and finished it! He finished that honors two men, Tom Redmond and “Hal” Quigley and Barbara (Young) Quigley third of the six men who were in the age-70- Jake McKenzie, who were lynched on Sloss visited Flora (Robinson) Hullstrung in and-over group. For that he received a $10 property in 1890 and 1897. In a “small August this past summer. All are doing well. gift certificate. Ruth (Schubert) Haynes world” moment, the man next to me and The late Bob Hullstrung’s cousin Garrett didn’t enter the race, but she is enjoying the I began the normal kind of conversation visited Flora for four days as well. Garrett new storage areas added to their kitchen. between two strangers. When it got around lives on Maui, Hawaii. Lynn (Swader) Monk Mary (Henck) Sharp modestly reported to children, he mentioned that his daughter Fedor is planning to move to a smaller that she and Richard have visited all 50 went to a small college in New Jersey. It facility. In the process of getting ready for states and seven continents. In August, they turned out to be Drew! And she was there! the move, she discovered her file of past went on a two-week truck tour of Russia. -

2018 DVJC Slalom
The Jaguar’s Purr© Is an official publication of THE DELAWARE VALLEY JAGUAR CLUB A chartered, non-profit corporation Founded in 1965 and incorporated in 1968. ©copyright 2018 All rights reserved. Reproduction without permission is prohibited. July 2018 2018 DVJC Slalom The 14th Annual DVJC Slalom will be held on Saturday, July 21, 2018, at the Garnet Valley High School, home of the Jaguars. Please see pages 9 & 10 for more information. One of our most consistent competitors was Kurt Rappold. Kurt could be counted on to bring something interesting to the event. The last several years he made his slalom circuits in a MK X, the only one in the country to compete in the slalom. Coming to this event, as a competitor or a volunteer, would be a great way to honor Kurt’s memory. The Jaguar’s Purr July 2018 Newsletter Contents Advertising Rates ....................................... 3 List of Officers ............................................ 3 Upcoming DVJC Events ........................... 4 Other Interesting Events .......................... 4 President’s Mewsings ................................ 5 DVJC 14th Annual Slalom.................... 9-10 Speaking of Things Jaguar ........................ 11 Are You Missing Out ............................... 16 DVJC Breakfast Socials ............................ 18 Classifieds ................................................. 19 DVJC Governance .................................... 19 NOTICE—It’s not too late to renew your DVJC membership for 2018. The membership fee is $65.00. If all your information is the same as last year please feel free to send a check to Ann Perry made out to DVJC. If any of your information has changed please let Ann Perry know of the changes. Please remember the membership directory and list- ing of vehicles owned is shared only with active members. -

COLLECTIVE INTELLIGENCE: Creating a Prosperous World at Peace
COLLECTIVE INTELLIGENCE: Creating a Prosperous World at Peace Foreword by Yochai Benkler (Re-mixed by Hassan Masum) The Wealth of Networks: Remixed Highlights Prefaces by Thomas Malone, Tom Atlee, & Pierre Lévy Edited by Mark Tovey Afterword by The Rt. Hon. Paul Martin & Thomas Homer-Dixon The Internet and the Revitalization of Democracies Earth Intelligence Network Oakton, Virginia Copyright © 2008, Earth Intelligence Network (EIN) This work is licensed under the Creative Commons Attribution-Noncommercial 3.0 Unported License For details go to http://creativecommons.org/licenses/by-nc/3.0/us/ EIN retains commercial and revenue rights. Authors retain other rights offered under copyright. Entire book and individual chapters free online at www.oss.net/CIB. Books available by the box of 20 at 50% off retail ($39.95). Published by Earth Intelligence Network February 2008 Post Office Box 369, Oakton, Virginia 22124 www.earth-intelligence.net Cover graphic combines two photographs from the National Aeronautical and Space Administration (NASA). The Earth by itself is available in a round color sticker as Item Apollo 17(E) from EarthSeals, POB 8000, Berkeley, CA 94707. Printed and bound in the United States of America 9 8 7 6 5 4 3 2 LIBRARY OF CONGRESS CATALOGING-IN-PUBLICATION DATA Tovey, Mark, 1970- COLLECTIVE INTELLIGENCE: Creating a Prosperous World at Peace/Mark Tovey (Editor) p. cm. Includes bibliographical references and index. ISBN 13: 978-0-9715661-6-3 (alk. paper) ISBN-10 0-9715661-6-X (alk. paper) 1. Collective intelligence. 2. Cognitive science. 3. Mass collaboration. 4. Distributed cognition. 5. Macrocognition. 6. Open source. -

B.E. Computer Science and Engineering
ANNA UNIVERSITY CHENNAI CHENNAI - 600 025 UNIVERSITY DEPARTMENTS REGULATIONS 2012 CURRICULA AND SYLLABI FOR I TO VIII SEMESTERS B.E. COMPUTER SCIENCE & ENGINEERING (FULL TIME) ANNA UNIVERSITY:: CHENNAI - 600 025. UNIVERSITY DEPARTMENT R - 2012 B.E. COMPUTER SCIENCE & ENGINEERING I – VIII SEMESTERS CURRICULA AND SYLLABI SEMESTER I Course Code Course Title L T P C THEORY HS8151 Technical English I 3 1 0 4 MA8151 Mathematics I 3 1 0 4 PH8151 Engineering Physics 3 0 0 3 CY8151 Engineering Chemistry 3 0 0 3 GE8151 Computing Techniques 3 0 0 3 GE8152 Engineering Graphics 2 0 3 4 PRACTICAL PH8161 Physics Laboratory 0 0 2 1 CY8161 Chemistry Laboratory 0 0 2 1 GE8161 Computer Practices Laboratory 0 0 3 2 GE8162 Engineering Practices Laboratory 0 0 3 2 TOTAL CREDITS 17 2 13 27 2 SEMESTER II Course code Course Title L T P C THEORY HS8251 Technical English II 3 1 0 4 MA8251 Mathematics II 3 1 0 4 PH8253 Physics for Information Science 3 0 0 3 CS8201 Digital Principles and System Design 3 0 0 3 CS8202 Principles of Computer Engineering 3 0 0 3 CS8203 Programming using C++ 3 0 0 3 PRACTICAL CS8211 Digital Laboratory 0 0 3 2 CS8212 Programming Laboratory 0 0 3 2 TOTAL CREDITS 18 2 6 24 SEMESTER III Course Code Course Title L T P C THEORY MA8351 Algebra and Number Theory 3 1 0 4 GE8351 Environmental Science and Engineering 3 0 0 3 CS8301 Computer Architecture 3 1 0 4 CS8302 Data Structures 3 0 0 3 CS8303 Database Management Systems 3 0 0 3 Electronic Devices and Circuits For Computer EC8303 3 0 0 3 Engineers PRACTICAL CS8311 Data Structures Laboratory -

Sci Report Frontmatter EP2:Layout 1
Strategic Corporate Initiative Toward a Global Citizens’ Movement to Bring Corporations Back Under Control Strategic Corporate Initiative Toward a Global Citizens’ Movement to Bring Corporations Back Under Control Michael Marx Mari Margil Corporate Ethics International John Cavanagh Sarah Anderson Chuck Collins Institute for Policy Studies Charlie Cray Center for Corporate Policy Marjorie Kelly Tellus Institute May 2007 Acknowledgments This work would not have been possible without the generous support of the Nathan Cummings Foundation and the Panta Rhea Foundation. The team wishes to thank the many activists, theorists, and executives who participated in meetings and interviews with us over the past year and a half, and who helped review and provide feedback for this report. In Appendix A we provide a full list of meeting and interview participants. We give special thanks to Jonathan Frieman, whose support and deep involvement have been invaluable. In addition, we would like to thank others who contributed to this project, including Gar Alperovitz, Harriet Barlow, Peter Barnes, Walden Bello, David Bollier, Dan Brannen, Marcia Carroll, Karen Hansen-Kuhn, Paul Hawken, Scott Klinger, David Korten, Harry Lonsdale, David Morris, John Richard, Julie Ristau, Joel Rogers, Rich Rosen, Kavaljit Singh, Mike Wallace, Rob Weissman, and Allen White. Our acknowledgment of their contribution does not mean that they endorse these findings, but rather that they took the time to share their opinions and insights. Design by Bryan Potter Design. Corporate Ethics International 720 SW Washington St., Suite 660 Portland, OR 97205 Phone 503/478-0888 www.CorporateEthics.org This is a final draft. Not for distribution. TABLE OF TABLE contents Overview Section 1: A Call for a Global Movement .