Classnotes: Fall 2019 Written by Kmerusi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

British Columbia
GIFT GUIDE 2018 BRITISH COLUMBIA Support Your Local Independent Bookseller Enjoy perusing this selection of books written by B.C. authors and titles published by B.C.’s many fine publishers. Stop by to explore the titles featured here—and discover a vast assortment of B.C. books that will meet your interests and needs. All prices listed are the publishers’ most up-to-date suggested retail prices available at the time of the printing of this gift guide. Publishers make every effort to hold these prices, but some changes may be necessary. The Death and Life of Strother Purcell Ian Weir The return of the Western—with a definite Cover image (Crow Jazz) Canadian twist. A deadpan revisionist from Crow Jazz Western for fans of Ron Rash and Cormac by Linda Rogers. McCarthy, The Death and Life of Strother Published by Mother Purcell is about two brothers, a pair of Tongue Publishing Limited. eldritch orphans, the vexed nature of truth Illustration copyright and the yearnings of that treacherous @ 2018 by Rick Van Krugel sonofabitch, the human heart. $22.95 pb Used by permission. 380 pp. 978-1-773100-29-6 (Goose Lane Editions) bc The Home for Wayward Parrots Washington Black Straight Circles Darusha Wehm Esi Edugyan Jackie Bateman Accustomed to being an only child, adoptee A dazzling, original novel of slavery and freedom Domestic satire meets gripping suspense in Brian “Gumbo” Guillemot’s hobby was searching by the author of the international bestseller the final, explosive chapter of Jackie Bateman’s for his birth parents. But when he finally finds Half-Blood Blues. -

PRESENTATIONS CANOECOPIA PRESENTATIONS for 2020 We Proudly Offer up a Cornucopia of Canoecopia Speakers & Topics
PRESENTATIONS CANOECOPIA PRESENTATIONS FOR 2020 We proudly offer up a cornucopia of Canoecopia speakers & topics. Christopher Amidon Paddling Isle Royale National Park Sat 1:30p, Quetico Sun 2:30p, Superior Isle Royale National Park offers unique opportunities for paddling in and around a wilderness island in Lake Superior. The challenges facing paddlers are many, from the logistics of transporting paddling equipment to the unpredictable and cold waters of Lake Superior. Join Ranger Chris Amidon to explore the paddling options and obstacles of Isle Royale National The Aluminum Chef Competition Park. Brought to you by MSR Sat 4:30p, Quetico Gregory Anderson Once again, our three Aluminum Chefs will test their camp culinary The Science of Waves skills against each other in true outdoor style. Kevin Callan returns as Fri 6:30p, Loon our unstoppable emcee in this fast-paced event. Woods-woman, Mona Have you ever wondered why wave fronts Gauthier and former park ranger Marty Koch go up against local chef end up parallel to the beach? How do shoals Luke Zahm of the Driftless Cafe in Viroqua, WI. Using MSR stoves create larger waves? Why do waves bend and cook kits, and a pantry of simple ingredients you might have on around obstacles? Understanding waves will your next camping trip (donated by the Driftless Cafe), our chefs will help you manage the surf zone and make you compete for the best appetizer, entree, and dessert. Come join the fun a better paddler, whether you want to avoid a - you could be one of the judges from the audience who will determine pounding or to catch the ride of your life. -

Elton John Tumbleweed Connection Mp3, Flac, Wma
Elton John Tumbleweed Connection mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Rock / Pop Album: Tumbleweed Connection Country: Japan Released: 2001 Style: Ballad, Classic Rock MP3 version RAR size: 1542 mb FLAC version RAR size: 1114 mb WMA version RAR size: 1806 mb Rating: 4.2 Votes: 445 Other Formats: WMA MIDI AUD MP4 APE XM ASF Tracklist Hide Credits Ballad Of A Well Known Gun Acoustic Guitar, Lead Guitar – Caleb QuayeBacking Vocals – Dusty Springfield, Kay A1 4:59 Garner, Lesley Duncan, Madeline Bell, Tony Burrows, Tony HazzardBass Guitar – Dave Glover*Drums, Percussion – Roger PopePiano – Elton John Come Down In Time A2 Acoustic Bass – Chris LaurenceAcoustic Guitar – Les ThatcherBass Guitar – Herbie 3:25 FlowersDrums – Barry MorganHarp – Skaila KangaOboe – Karl Jenkins Country Comfort Acoustic Guitar – Caleb QuayeAcoustic Guitar [12-String] – Les ThatcherBacking Vocals – A3 5:08 Dee Murray, Nigel OlssonBass Guitar – Herbie FlowersDrums – Barry MorganHarmonica – Ian DuckPiano – Elton JohnSteel Guitar – Gordon HuntleyViolin – Johnny Van Derek Son Of Your Father Backing Vocals – Kay Garner, Lesley Duncan, Madeline Bell, Sue And Sunny*, Tammi A4 3:46 HuntBass Guitar – Dave Glover*Drums – Roger PopeHarmonica – Ian DuckLead Guitar – Caleb QuayePiano – Elton John My Father's Gun Backing Vocals – Dusty Springfield, Kay Garner, Lesley Duncan, Madeline Bell, Tony A5 6:19 Burrows, Tony HazzardBass Guitar – Dave Glover*Drums – Roger PopeElectric Guitar, Acoustic Guitar – Caleb QuayePiano – Elton John Where To Now St. Peter B1 Backing Vocals -

Which Would Niirt1 Vibilmlese Score
WATER CONDITION 0i)uuttlftntnaTEDPY'S TIDES 0~ztttt HIGH LOW CHARLIE V a.m. 5:28 p.m. U. S. NAVAL BASE, GUANTANAMO BAY, CUBA 14 SIO GALLO 11:11 p.m. 3:34 p.m. 14 LlON G Systems 'Go' for Apollo 1L Russian Rocket May Return Thieu Pro roses Nati0n81 Elections With Samples of Moon Soil CAPE kENNEDY, Fla.(AP/AFRTS) SAIGON (UPI/AFRTS)-- South Vietnamese President Thieu has -- All systems are "go" for proposed a new step toward ending the fighting in that country. the Apollo 11 moon landing He called for free elections, saying that the Viet Cong could flight. participate if they agree to denounce violence. Apollo launch director Rocco Thieu's plan also called for establishment of an election Petrone says everything is in commission with all political factions. He said he was will- very fine shape regarding op- ing to discuss an election timetable with the other side. He erations and the status of the did not specify what the elections would be for, but it is be- hardware. Even the weatherman lieved he wants only Presidential and House elections. has kept things on an even The Viet Cong wants election keel with a forecast for fav- of an assembly which would NIirt1 Vibilmlese orable conditions Wednesday. work out a new constitution Score As the astronauts took yes- and then election of a coali- ' terday off and the ground tion government. lIAHms Race 0is crews enjoyed a 16-hour rest President Nixon has issued a SAIGON (AP/AFRTS) -- Banoi period, Russia moved in on lengthy statement hailing charged yesterday that des- some of the space limelight. -
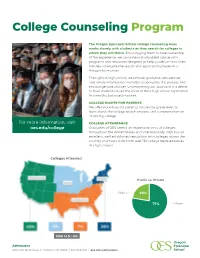
College Counseling Program
College Counseling Program The Oregon Episcopal School college counseling team works closely with students as they search for colleges in which they will thrive. Encouraging them to take ownership of the experience, we combine individualized advice with programs and resources designed to help students—and their families—navigate the search and application phases in a thoughtful manner. Throughout high school, we provide guidance, perspective, and timely information intended to demystify the process and encourage wise choices. Underpinning our approach is a desire to have students make the most of their high school experience in a healthy, balanced manner. COLLEGE NIGHTS FOR PARENTS We offer workshops for parents, tailored by grade level, to learn about the college search process, and a presentation on financing college. For more information, visit: COLLEGE ATTENDANCE oes.edu/college Graduates of OES attend an impressive array of colleges throughout the United States and internationally. OES has an excellent, well-established reputation with colleges across the country and hosts visits from over 130 college representatives in a typical year. Colleges Attended Public vs. Private Public 29% 71% Private Non U.S.: 4% Admissions 6300 SW Nicol Road | Portland, OR 97223 | 503-768-3115 | oes.edu/admissions OES STUDENTS FROM THE CLASSES OF 2020 AND 2021 WERE ACCEPTED TO THE FOLLOWING COLLEGES Acadia University Elon University Pomona College University of Chicago Alfred University Emerson College Portland State University University of Colorado, -

The Drug Repurposing Ecosystem: Intellectual Property Incentives
Halabi: The Drug Repurposing Ecosystem: Intellectual Property Incentives, The Drug Repurposing Ecosystem: Intellectual Property Incentives, Market Exclusivity, and the Future of "New" Medicines Sam F. Halabil 20 YALE J. L. & TECH. 1 (2018) The pharmaceutical industry is in a state of fundamental transition.New drug approvals have slowed, patents on blockbuster drugs are expiring, and costs associated with developing new drugs are escalating and yielding fewer viable drug candidates. As a result, pharmaceutical firms have turned to a number of alternative strategies for growth. One of these strategies is "drug rep urposing"-findingnew ways to deploy approved drugs or abandoned clinical candidates in new disease areas. Despite the efficiency advantages of repurposing drugs, there is broad agreement that there is insufficient repurposing activity because of numerous intellectual property protection and market failures. This Article examines the system that surrounds drug repurposing, including serendipitous discovery, the application of "big data" methods to prioritize promising repurposing candidates, the unorthodoxly regulated off-label prescription practices of providers, and related prohibitions on pharmaceutical firms' off-label marketing. The Article argues that there is a complex ecosystem in place and that additional or disruptive IP Fulbright Canada Research Chair in Health Law, Policy, and Ethics, University of Ottawa and Associate Professor, University of Missouri School of Law.; J.D. Harvard; M.Phil. The University of Oxford (St. Antony's College); B.A., B.S. Kansas State University. The author is grateful for comments and helpful suggestions given at faculty workshops at Arizona State University's Sandra Day O'Connor School of Law, Boston University's biolP Faculty Workshop, and Seton Hall University School of Law, as well as the American Society of Law, Medicine, and Ethics' Annual Health Law Professors Conference. -

The Office of College Counseling Nancy Thatcher College Counselor
The Office of College Counseling Nancy Thatcher College Counselor What We (all) Do From here To here The General Process • Presentation to 8th grade by counselor and current 9th grade students • Aspire, PSAT testing and general college guidance for 9th-10th grades • SAT/ACT testing • Junior College Prep class, Spring of Junior year • Senior College Prep class, Fall of Senior year • Continuous dialogue throughout high school among all faculty/students around college Family Connection • Back to School Night/Ninth Grade orientation Night • Junior College Night • Senior College Night • Financial Aid Night • Senior Wrap-Up Evening • Student-Parent College meetings • Alumni Panel How Do We Compare? GHCDS Antilles School • 2013-2015 Matriculation • Iona College • Smith College • 2013-2015 Matriculation Technology • Worchester Polytechnic • Agnes Scott College • Iowa State University • Stanford University • College Acceptances • New York University • Institute • Allegheny College • Ithaca College • Stetson University • Northeastern University • Yale University • American University • Jacksonville University • St. John College • American University • Nova Southeastern • Amherst College • Johnson and Wales University • St. Lawrence University • Agnes Scott College University • Aquinas College • Lafayette College • St. Peter’s College • Babson College • Ohio Wesleyan University • Babson College • LaRoche College • Suffolk University • Bentley University • Providence College • Bard College • LaSalle University • Southern Methodist • Bowdoin College -

Parsons College Alumni Website
Parsons College E-News Volume 9, No. 1 Spring - 2016 Springtime spawns memories of America’s #1 pastime..BASEBALL! PARSONS COLLEGE BASEBALL – ONE OF THE TOP COLLEGIATE PROGRAMS OF THE 1960’S WITH A .814 WINNING PERCENTAGE OVER SEVEN SEASONS Parsons College in the 1960’s was well known for its top academics, social life and athletic programs. This spring, we’re highlighting the baseball teams that carried a winning banner throughout the Midwest and the nation. The seven seasons between 1964 and 1970 the Wildcat team won 233 games and lost only 53 games for a .814 winning average. During those years they also sent a number of players off to Major League Baseball including pitchers, Rich Folkers, Charlie Williams, Dick Mills and Jim Todd, to name a few. The Coaches who made it happen!!! Coach Joe Lutz got the program rolling in 1964 Coach Hall, 1966 Coach Banks, 1966-69 Coach Blixt, 1970-73 Dr. Millard G. Roberts vision for national recognition extended beyond the academic halls of campus. He was determined to send a message to future students that Parsons College was a well-rounded institution. He made that very clear when his athletic department extended an invitation to Coach Joe Lutz in the fall of 1964 to take over and develop a top notch varsity baseball program. Lutz was born on February 18, 1925, in Keokuk, Iowa and was a high school baseball standout. He signed a professional contract with the St. Louis Browns in 1941. Joe played for minor league teams in the Browns organization and advanced to their AAA level in1951 prior to being traded to the Brooklyn Dodgers organization. -

FICE Code List for Colleges and Universities (X0011)
FICE Code List For Colleges And Universities ALABAMA ALASKA 001002 ALABAMA A & M 001061 ALASKA PACIFIC UNIVERSITY 001005 ALABAMA STATE UNIVERSITY 066659 PRINCE WILLIAM SOUND C.C. 001008 ATHENS STATE UNIVERSITY 011462 U OF ALASKA ANCHORAGE 008310 AUBURN U-MONTGOMERY 001063 U OF ALASKA FAIRBANKS 001009 AUBURN UNIVERSITY MAIN 001065 UNIV OF ALASKA SOUTHEAST 005733 BEVILL STATE C.C. 001012 BIRMINGHAM SOUTHERN COLL ARIZONA 001030 BISHOP STATE COMM COLLEGE 001081 ARIZONA STATE UNIV MAIN 001013 CALHOUN COMMUNITY COLLEGE 066935 ARIZONA STATE UNIV WEST 001007 CENTRAL ALABAMA COMM COLL 001071 ARIZONA WESTERN COLLEGE 002602 CHATTAHOOCHEE VALLEY 001072 COCHISE COLLEGE 012182 CHATTAHOOCHEE VALLEY 031004 COCONINO COUNTY COMM COLL 012308 COMM COLLEGE OF THE A.F. 008322 DEVRY UNIVERSITY 001015 ENTERPRISE STATE JR COLL 008246 DINE COLLEGE 001003 FAULKNER UNIVERSITY 008303 GATEWAY COMMUNITY COLLEGE 005699 G.WALLACE ST CC-SELMA 001076 GLENDALE COMMUNITY COLL 001017 GADSDEN STATE COMM COLL 001074 GRAND CANYON UNIVERSITY 001019 HUNTINGDON COLLEGE 001077 MESA COMMUNITY COLLEGE 001020 JACKSONVILLE STATE UNIV 011864 MOHAVE COMMUNITY COLLEGE 001021 JEFFERSON DAVIS COMM COLL 001082 NORTHERN ARIZONA UNIV 001022 JEFFERSON STATE COMM COLL 011862 NORTHLAND PIONEER COLLEGE 001023 JUDSON COLLEGE 026236 PARADISE VALLEY COMM COLL 001059 LAWSON STATE COMM COLLEGE 001078 PHOENIX COLLEGE 001026 MARION MILITARY INSTITUTE 007266 PIMA COUNTY COMMUNITY COL 001028 MILES COLLEGE 020653 PRESCOTT COLLEGE 001031 NORTHEAST ALABAMA COMM CO 021775 RIO SALADO COMMUNITY COLL 005697 NORTHWEST -
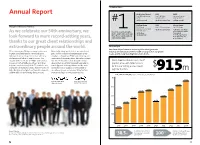
Annual Report
Top Ranking Report Annual Report Architectural Record ENR VMSD Top 300 Architecture Top 150 Global Top Retail Design Firms: Design Firms: Firms of 2014: # #1 Firm Overall #1 Architecture Firm #1 Firm Overall Building Design ENR Interior Design Message from the Board of Directors 2014 World Top 500 Design Firms: Top 100 Giants: Architecture 100 Most #1 Architecture Firm #1 Architecture Firm Admired Firms: Gensler is1 a leader among the #1 in Corporate Office As we celebrate our 50th anniversary, we world’s architecture and design #1 US Firm #1 in Retail #4 Global Firm #1 in Transportation firms. Here’s how we ranked in #1 in Government look forward to more record-setting years, our industry in 2014. #1 in Cultural thanks to our great client relationships and extraordinary people around the world. Financial Report Our financial performance and recognition throughout the We’re entering our 50th year stronger than ever. Financially strong and debt-free, we contributed industry are indications of the breadth of our practice, our global In 2014, our global growth continued apace $38.5 million in deferred compensation to our reach, and the long-standing trust of our clients. with our clients as they entrusted us with new employees through our ESOP, profit-sharing, and challenges and led us to new locations. Our international retirement plans. We made strategic expanded Gensler team of 4,700+ professionals investments in our research and professional We’ve broadened our services to 27 now work from 46 different offices. With their development programs, along with upgrades to practice areas, with total revenues help, we completed projects in 72 countries and our design-and-delivery platform and the tools for the year setting a new record $ increased our revenues to $915 million—a record and technology to support it. -

ASA Official Rules of Softball Umpire Edition
Welcome! Pick here for General Table of Contents Pick here for Playing Rules Table of Contents On this information page you will find: General notes about this rulebook. Other Notes: General notes about this rulebook. SEARCH: This rulebook is presented using Adobe Acrobat®. This allows you the user to search the rulebook for specific text using the Adobe Acrobat®software search tool. Select the binocular icon, type in the word or phrase you are looking for and pick the search button. NAVIGATION: You may navigate the rulebook using the bookmarks shown on the left or from either of the Table of Contents pages. To Navigate using the bookmarks simply select the title of the bookmark and the Acrobat software will take you to that page. To navigate from a Table of Contents page simply select the title or page number you wish to go to. RETURNING TO THE UMPIRE MECHANICS SOFTWARE: The umpire mechanics software is still running when you launch this rulebook. This allows you to switch between the umpire mechanics software and this rulebook. There are several ways to accomplish this on a windows machine. Most commonly the taskbar at the bottom of the screen or using the “Alt” and “Tab” key to cycle through the software programs that are running on your computer. SOFTBALL PLAYING RULES Copyright by the Amateur Softball Association of America REVISED 2005 “Permission to reprint THE OFFICIAL PLAYING RULES has been granted by THE AMATEUR SOFTBALL ASSOCIATION OF AMERICA.” Where (Fast Pitch Only) is shown, Modified Pitch rules are followed the same as fast pitch with the exception of the pitching rule. -

The Replay News 1930 FINAL EDITION
The Replay News 1930 FINAL EDITION MVP’s Lefty Grove (Top) and Chuck Klein Table of Contents 3- Final Standings 4- American League Batting Leaders 5- American League Pitching Leaders 6- National League Batting Leaders 7- National League Pitching Leaders 8- Team-by-Team Individual Batting and Pitching Stats 24- Team Batting and Pitching Stats 25- Top Game Performances 26- World Series Summary 27- World Series Scoresheets 32- Comparison of Individual Batters’ Stats to Actual 46- Comparison of Individual Pitchers’ Stats to Actual MLB Standings Through Games Of 9/28/1930 American League W LGB Pct Strk R RA Philadelphia Athletics 105 49-- .682 W1 969 639 Washington Senators 97 578.0 .630 L1 882 685 New York Yankees 92 6213.0 .597 W3 1105 881 Detroit Tigers 78 7627.0 .506 L2 772 802 Cleveland Indians 67 8738.0 .435 W1 781 929 Chicago White Sox 65 8940.0 .422 W2 760 886 Boston Red Sox 60 9445.0 .390 L3 672 859 St. Louis Browns 52 10253.0 .338 L1 687 947 National League W LGB Pct Strk R RA Chicago Cubs 98 56-- .636 W3 961 781 New York Giants 89 659.0 .578 L3 909 793 Pittsburgh Pirates 85 6913.0 .552 L1 960 888 Brooklyn Robins 83 7115.0 .539 W2 876 774 St. Louis Cardinals 83 7115.0 .539 W1 980 828 Philadelphia Phillies 64 9034.0 .416 W4 977 1223 Boston Braves 59 9539.0 .383 L2 724 848 Cincinnati Reds 55 9943.0 .357 L3 723 954 American League Leaders Including Games of Sunday, September 28, 1930 Hits Strikeouts Batting Leaders Lou GehrigNYA 239 Tony LazzeriNYA 70 Carl ReynoldsCHA 224 Ed MorganCLE 69 Batting Average Al SimmonsPHA 223 Jimmie FoxxPHA