On the Taxonomy and Systematics of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Radiation of a Dozen Species on Sky Islands Across Peninsular India (Squamata: Gekkonidae, Hemiphyllodactylus) with the Description of Three New Species
Organisms Diversity & Evolution (2019) 19:341–361 https://doi.org/10.1007/s13127-019-00392-5 ORIGINAL ARTICLE The hills are alive with geckos! A radiation of a dozen species on sky islands across peninsular India (Squamata: Gekkonidae, Hemiphyllodactylus) with the description of three new species Ishan Agarwal1,2 & Akshay Khandekar1,2 & Varad B. Giri1,3 & Uma Ramakrishnan1 & K. Praveen Karanth 2 Received: 6 August 2018 /Revised: 9 January 2019 /Accepted: 12 February 2019 /Published online: 5 March 2019 # Gesellschaft für Biologische Systematik 2019 Abstract Sky Islands are high-elevation environments that are separated by warmer, low elevations, forming natural patches of unique montane habitat that often persist through changing climates. Peninsular India was ancestrally forested and has gradually become more arid since at least the Oligocene, and open landscapes have dominated since the middle-late Miocene. Mesic forests today are largely restricted to coastal mountains and some other montane habitats. A mitochondrial phylogeny and fossil-calibrated timetree of Indian Hemiphyllodactylus reveal an Indochinese origin and an endemic radiation with 12 species-level lineages, where a single species was known, that diversified in the Oligocene-Miocene across montane forest habitats in the Eastern Ghats and south India. The phylogeny also suggests the discontinuous Eastern Ghats mountain range encompasses two distinct biogeographic entities: north and south of the Pennar/Krishna-Godavari River basins. This study highlights the deep history of the region and the importance of montane habitats as islands of unique biodiversity that have persisted through millions of years of changing climates. We describe three new species: Hemiphyllodactylus arakuensis sp. -

Unsustainable Food Systems Threaten Wild Crop and Dolphin Species
INTERNATIONAL PRESS RELEASE Embargoed until: 07:00 GMT (16:00 JST) 5 December 2017 Elaine Paterson, IUCN Media Relations, t+44 1223 331128, email [email protected] Goska Bonnaveira, IUCN Media Relations, m +41 792760185, email [email protected] [In Japan] Cheryl-Samantha MacSharry, IUCN Media Relations, t+44 1223 331128, email [email protected] Download photographs here Download summary statistics here Unsustainable food systems threaten wild crop and dolphin species Tokyo, Japan, 5 December 2017 (IUCN) – Species of wild rice, wheat and yam are threatened by overly intensive agricultural production and urban expansion, whilst poor fishing practices have caused steep declines in the Irrawaddy Dolphin and Finless Porpoise, according to the latest update of The IUCN Red List of Threatened Species™. Today’s Red List update also reveals that a drying climate is pushing the Ringtail Possum to the brink of extinction. Three reptile species found only on an Australian island – the Christmas Island Whiptail-skink, the Blue- tailed Skink (Cryptoblepharus egeriae) and the Lister’s Gecko – have gone extinct, according to the update. But in New Zealand, conservation efforts have improved the situation for two species of Kiwi. “Healthy, species-rich ecosystems are fundamental to our ability to feed the world’s growing population and achieve the UN Sustainable Development Goal 2 – to end hunger by 2030,” says IUCN Director General Inger Andersen. “Wild crop species, for example, maintain genetic diversity of agricultural crops -

ONEP V09.Pdf
Compiled by Jarujin Nabhitabhata Tanya Chan-ard Yodchaiy Chuaynkern OEPP BIODIVERSITY SERIES volume nine OFFICE OF ENVIRONMENTAL POLICY AND PLANNING MINISTRY OF SCIENCE TECHNOLOGY AND ENVIRONMENT 60/1 SOI PIBULWATTANA VII, RAMA VI RD., BANGKOK 10400 THAILAND TEL. (662) 2797180, 2714232, 2797186-9 FAX. (662) 2713226 Office of Environmental Policy and Planning 2000 NOT FOR SALE NOT FOR SALE NOT FOR SALE Compiled by Jarujin Nabhitabhata Tanya Chan-ard Yodchaiy Chuaynkern Office of Environmental Policy and Planning 2000 First published : September 2000 by Office of Environmental Policy and Planning (OEPP), Thailand. ISBN : 974–87704–3–5 This publication is financially supported by OEPP and may be reproduced in whole or in part and in any form for educational or non–profit purposes without special permission from OEPP, providing that acknowledgment of the source is made. No use of this publication may be made for resale or for any other commercial purposes. Citation : Nabhitabhata J., Chan ard T., Chuaynkern Y. 2000. Checklist of Amphibians and Reptiles in Thailand. Office of Environmental Policy and Planning, Bangkok, Thailand. Authors : Jarujin Nabhitabhata Tanya Chan–ard Yodchaiy Chuaynkern National Science Museum Available from : Biological Resources Section Natural Resources and Environmental Management Division Office of Environmental Policy and Planning Ministry of Science Technology and Environment 60/1 Rama VI Rd. Bangkok 10400 THAILAND Tel. (662) 271–3251, 279–7180, 271–4232–8 279–7186–9 ext 226, 227 Facsimile (662) 279–8088, 271–3251 Designed & Printed :Integrated Promotion Technology Co., Ltd. Tel. (662) 585–2076, 586–0837, 913–7761–2 Facsimile (662) 913–7763 2 1. -

Survival Method and Habitat of Common Wolf Snake Dr
International Journal of Engineering, Science and Mathematics Vol. 6 Issue 3, July 2017, ISSN: 2320-0294 Impact Factor: 6.765 Journal Homepage: http://www.ijesm.co.in, Email: [email protected] Double-Blind Peer Reviewed Refereed Open Access International Journal - Included in the International Serial Directories Indexed & Listed at: Ulrich's Periodicals Directory ©, U.S.A., Open J-Gage as well as in Cabell’s Directories of Publishing Opportunities, U.S.A Survival method and habitat of Common Wolf snake Dr. Pushpa Kumari PhD. (Zoology) Calorx Teachers' University, Ahmedabad Abstract A new species of the genus Lycodon Fitzinger, 1826 is described from the Cardamom Mountains of southwest Cambodia.Lycodon zoosvictoriae distinctly differs from all other species of Lycodon in Southeast Asia by a combination of its morphometric characters and unique coloration. The new species has 17 dorsal scales at midbody; 2+2 temporals; 8 supralabials; 10 infralabials; loreal separated from internasal and orbit; 213 ventrals; 85 subcaudals; pale tan brown ground color,irregular dark brown blotches on anterior part, 31 transverse blotches on posterior part of body and 26 blotches on tail.Given its submontane type locality, the new species could prove to be endemic to the Cardamom Mountains of southwestCambodia and probably Southeast Thailand. Key words: herpetofauna, Pursat, Indochina, Lycodon Introduction A new species of wolf snake is described from the tropical mixed dry deciduous forests of the Anaikatti Hills, Western Ghats, India. This species is distinct from its congeners of the Indian mainland by the following combination of characters: dorsal scales in 17:17:15 rows, scales smooth with single apical pit; anterior nasal shield larger than the posterior, loreal in contact with the internasal, but not with the eye; higher number of ventrals (210 - 224) which do not angulate laterally and hemipenis not forked at the tip and lacks spines. -

The Contribution of Policy, Law, Management, Research, and Advocacy Failings to the Recent Extinctions of Three Australian Vertebrate Species
This is the peer reviewed version of the following article: Woinarski, J. C. Z., Garnett, S. T., Legge, S. M., & Lindenmayer, D. B. (2017). The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species. Conservation Biology, 31(1), 13-23; which has been published in final form at http://doi.org/10.1111/cobi.12852 This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species John C.Z. Woinarski,*,a Stephen T. Garnett,* Sarah M. Legge,* † David B. Lindenmayer ‡ * Threatened Species Recovery Hub of the National Environment Science Programme, Research Institute for the Environment and Livelihoods, Charles Darwin University, Darwin, Northern Territory 0909, Australia, † Threatened Species Recovery Hub of the National Environment Science Programme, University of Queensland, St Lucia, Queensland 4072, Australia ‡ Threatened Species Recovery Hub of the National Environment Science Programme, Fenner School of Environment and Society, The Australian National University, Canberra, ACT 2601, Australia aemail [email protected] Keywords: Bramble Cay melomys, Christmas Island forest skink, Christmas Island pipistrelle, conservation policy, inquest, legislation, threatened species Running head: Extinction contributing factors Abstract Extinctions typically have ecological drivers, such as habitat loss. However, extinction events are also influenced by policy and management settings that may be antithetical to biodiversity conservation, inadequate to prevent extinction, insufficiently resourced, or poorly implemented. Three endemic Australian vertebrate species – the Christmas Island pipistrelle (Pipistrellus murrayi), Bramble Cay melomys (Melomys rubicola), and Christmas Island forest skink (Emoia nativitatis) – became extinct from 2009 to 2014. -

New Records of Snakes (Squamata: Serpentes) from Hoa Binh Province, Northwestern Vietnam
Bonn zoological Bulletin 67 (1): 15–24 May 2018 New records of snakes (Squamata: Serpentes) from Hoa Binh Province, northwestern Vietnam Truong Quang Nguyen1,2,*, Tan Van Nguyen 1,3, Cuong The Pham1,2, An Vinh Ong4 & Thomas Ziegler5 1 Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam 2 Graduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Road, Hanoi, Vietnam 3 Save Vietnam’s Wildlife, Cuc Phuong National Park, Ninh Binh Province, Vietnam 4 Vinh University, 182 Le Duan Road, Vinh City, Nghe An Province, Vietnam 5 AG Zoologischer Garten Köln, Riehler Strasse 173, D-50735 Cologne, Germany * Corresponding author. E-mail: [email protected] Abstract. We report nine new records of snakes from Hoa Binh Province based on a reptile collection from Thuong Tien, Hang Kia-Pa Co, Ngoc Son-Ngo Luong nature reserves, and Tan Lac District, comprising six species of Colubri- dae (Dryocalamus davisonii, Euprepiophis mandarinus, Lycodon futsingensis, L. meridionalis, Sibynophis collaris and Sinonatrix aequifasciata), one species of Pareatidae (Pareas hamptoni) and two species of Viperidae (Protobothrops mu- crosquamatus and Trimeresurus gumprechti). In addition, we provide an updated list of 43 snake species from Hoa Binh Province. The snake fauna of Hoa Binh contains some species of conservation concern with seven species listed in the Governmental Decree No. 32/2006/ND-CP (2006), nine species listed in the Vietnam Red Data Book (2007), and three species listed in the IUCN Red List (2018). Key words. New records, snakes, taxonomy, Hoa Binh Province. -

Occurrence and Distribution of Snake Species in Balochistan Province, Pakistan
Pakistan J. Zool., pp 1-4, 2021. DOI: https://dx.doi.org/10.17582/journal.pjz/20181111091150 Short Communication Occurrence and Distribution of Snake Species in Balochistan Province, Pakistan Saeed Ahmed Essote1, Asim Iqbal1, Muhammad Kamran Taj2*, Asmatullah Kakar1, Imran Taj2, Shahab-ud-Din Kakar1 and Imran Ali 3,4* 1Department of Zoology, University of Balochistan, Quetta 2Center for Advanced Studies in Vaccinology and Biotechnology, University of Balochistan, Quetta, Pakistan. 3Institute of Biochemistry, University of Balochistan, Quetta 4 Plant Biomass Utilization Research Unit, Chulalongkorn University, Bangkok, 10330, Article Information Thailand. Received 11 November 2018 Revised 11 October 2020 Accepted 10 December 2020 ABSTRACT Available online 28 April 2021 Authors’ Contribution The current study was conducted in Zhob, Quetta, Sibi, Kalat, Naseer Abad and Makran Divisions of SAE carried the research with the Balochistan Province. A total of 619 snake specimens representing 6 families, 20 genera and 37 species help of other authors and wrote were collected. The family wise representation among collected specimens has been Boidae (4.6%), the manuscript. AI, MKT and IT Leptotyphlopidae (7.5%), Typhlopidae (10.3%), Elapidae (11.7%), Viperidae (13.4%) and Colubridae helped in the experimental work. AK (52.5%). The percentage of family Boidae, Typhlopidae, Elapidae and Leptotyphlopidae were high in and SDK classified the species and Sibi Division while family Viperidae and Colubridae were dominant in Quetta Division. The family proofread the article. IA helped in arranging contents of the article. Colubridae has been the most dominant in the Province, having ten genera viz., Boiga (6.8%) Coluber (10.1%), Eirenis (2.5%), Lycodon (3.5%), Lytorhynchus (6.1%), Oligodon (4.7%), Natrix (1.7%), (7.5%), Key words Ptyas (2.9 %) Spalerosophis (6.3%) and Psammophis. -

Cfreptiles & Amphibians
HTTPS://JOURNALS.KU.EDU/REPTILESANDAMPHIBIANSTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANSREPTILES • VOL &15, AMPHIBIANS NO 4 • DEC 2008 • 28(1):157–158189 • APR 2021 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS FEATUREPredation ARTICLES on a Common Wolfsnake, . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: LycodonOn the Road to aulicusUnderstanding the Ecology (Colubridae),and Conservation of the Midwest’s Giant Serpent ...................... by anJoshua M. KapferIndian 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: Roller,A Hypothetical Coracias Excursion ............................................................................................................................ benghalensis (Coraciidae),Robert W. Henderson 198 RESEARCH ARTICLES in. The the Texas Horned Sathyamangalam Lizard in Central and Western Texas ....................... Emily Henry, JasonTiger Brewer, Krista Mougey, Reserve, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida .............................................TamilBrian J. Camposano, Kenneth Nadu, L. Krysko, Kevin M. Enge,India Ellen M. Donlan, and Michael Granatosky 212 CONSERVATION ALERT . World’s Mammals in Crisis ...............................................................................................................................Sreedharan Nair Vishnu and Chinnasamy Ramesh .............................. 220 . More Than Mammals ..................................................................................................................................................................... -
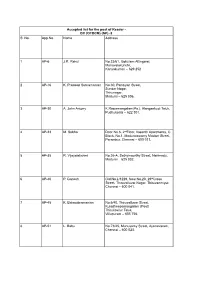
S. No. App.No. Name Address 1 AP-6 J.R. Rahul 2 AP-16 K. Pradeep
Accepted list for the post of Reader - BC (OTBCM) (NP) -2 S. No. App.No. Name Address 1 AP-6 J.R. Rahul No.23/61, Gokulam Attingarai, Manavalakurichi, Kanyakumari – 629 252 2 AP-16 K. Pradeep Subramanian No.30, Pandiyan Street, Sundar Nagar, Thirunagar, Madurai – 625 006. 3 AP-30 A. John Antony K.Rasiamangalam(Po.), Alangankudi Taluk, Pudhukottai – 622 301. 4 AP-33 M. Subha Door No.6, 2ndFloor, Vasanth Apartments, C Block, No.1, Maduraiswamy Madam Street, Perambur, Chennai – 600 011. 5 AP-35 R. Vijayalakshmi No.26-A, Sathymoorthy Street, Narimedu, Madurai – 625 002. 6 AP-40 P. Ganesh Old No.L/1229, New No.20, 29thCross Street, Thiruvalluvar Nagar, Thiruvanmiyur, Chennai – 600 041. 7 AP-45 K. Balasubramanian No.6/40, Thiruvalluvar Street, Kuladheepamangalam (Post) Thirukovilur Taluk, Villupuram – 605 756. 8 AP-51 L. Babu No.73/45, Munusamy Street, Ayanavaram, Chennai – 600 023. 9 AP-56 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Pondicherry – 605 007. 10 AP-62 R.D. Mathanram No.57, Jeeyar Narayanapalayam St, Kanchipuram – 631 501 11 AP-77 M.Parameswari No.8/4, Alagiri Nagar, 1ststreet, Vadapalani, chennai -26. 12 AP-83 G. Selva Kumari No. 12, G Block, Singara thottam, Police Quarters, Old Washermen pet, Chennai 600 021 13 AP-89 P. Mythili No.137/64, Sanjeeviroyan Koil Street, Old Washermenpet, Chennai – 600 021. 14 AP-124 K. Balaji No.11, Muthumariamman Koil Street, Bharath Nagar, Selaiyur, Chennai – 600 073. 15 AP-134 S. Anitha No.5/55-A, Main Road, Siruvangunam, Iraniyasithi Post, Seiyur Taluk, Kancheepuram – 603 312. -

Genus Lycodon)
Zoologica Scripta Multilocus phylogeny reveals unexpected diversification patterns in Asian wolf snakes (genus Lycodon) CAMERON D. SILER,CARL H. OLIVEROS,ANSSI SANTANEN &RAFE M. BROWN Submitted: 6 September 2012 Siler, C. D., Oliveros, C. H., Santanen, A., Brown, R. M. (2013). Multilocus phylogeny Accepted: 8 December 2012 reveals unexpected diversification patterns in Asian wolf snakes (genus Lycodon). —Zoologica doi:10.1111/zsc.12007 Scripta, 42, 262–277. The diverse group of Asian wolf snakes of the genus Lycodon represents one of many poorly understood radiations of advanced snakes in the superfamily Colubroidea. Outside of three species having previously been represented in higher-level phylogenetic analyses, nothing is known of the relationships among species in this unique, moderately diverse, group. The genus occurs widely from central to Southeast Asia, and contains both widespread species to forms that are endemic to small islands. One-third of the diversity is found in the Philippine archipelago. Both morphological similarity and highly variable diagnostic characters have contributed to confusion over species-level diversity. Additionally, the placement of the genus among genera in the subfamily Colubrinae remains uncertain, although previous studies have supported a close relationship with the genus Dinodon. In this study, we provide the first estimate of phylogenetic relationships within the genus Lycodon using a new multi- locus data set. We provide statistical tests of monophyly based on biogeographic, morpho- logical and taxonomic hypotheses. With few exceptions, we are able to reject many of these hypotheses, indicating a need for taxonomic revisions and a reconsideration of the group's biogeography. Mapping of color patterns on our preferred phylogenetic tree suggests that banded and blotched types have evolved on multiple occasions in the history of the genus, whereas the solid-color (and possibly speckled) morphotype color patterns evolved only once. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

Reptile Rap Newsletter of the South Asian Reptile Network ISSN 2230-7079 No.18 | November 2016 Date of Publication: 30 November 2016
Reptile Rap Newsletter of the South Asian Reptile Network No.18 | November 2016 ISSN 2230-7079 Date of publication: 30 November 2016 www.zoosprint.org/Newsletters/ReptileRap.htm OPEN ACCESS | FREE DOWNLOAD REPTILE RAP #18, 30 November 2016 Contents A pilot-survey to assess the diversity and distribution of reptilian fauna in Taralu Village, abutting the Bannerghatta National Park, Karnataka, India -- S. Aaranya Gayathri, M. Jayashankar & K. Avinash, Pp. 3–18 A comprehensive report on the Hook-nosed Sea Snake Enhydrina schistosa (Daudin, 1803) -- Hatkar Prachi & Chinnasamy Ramesh, Pp. 19–22 A sighting of the Sind Awl-headed Snake Lytorhynchus paradoxus (Günther, 1875) from western Rajasthan: Habitat preferences -- Kachhawa Yati, Kachhawa Dimple, Kumawat Kumar Rakesh, K.K. Sharma & Sharma Vivek, Pp. 23–24 Distribution of Treutler’s Gecko (Hemidactylus treutleri Mahony, 2009) in Telangana and Andhra Pradesh, southern India - a general information -- B. Laxmi Narayana, G. Baburao & V. Vasudeva Rao, Pp. 25–28 On the occurrence of the Calamaria Reed Snake Liopeltis calamaria (Günther, 1858) (Squamata: Colubridae), in the Kalakadu Mundanthurai Tiger Reserve, India -- Surya Narayanan, Pp. 29–30 Note on record of body length of the Common Wolf Snake Lycodon aulicus -- Raju Vyas, Pp. 31–32 Unusual feeding behavior of the Checkered Keelback Xenochrophis piscator on Jahangirnagar University Campus, Savar, Dhaka, Bangladesh -- Noman Al Moktadir & Md. Kamrul Hasan, Pp. 32–33 Bifid tail inHemidactylus prashadi (Smith, 1935) -- Shivanand R. Yankanchi & Suresh M. Kumbar, Pp. 34–35 Some observations on the Malabar Pit Viper Trimeresurus malabaricus in central Western Ghats, India -- Uday Sagar, Pp. 36–39 First records of Oligodon taeniolatus and Bungarus sindnus walli from Nagpur District, Maharashtra, India -- Deshmukh, R.V., Sager A.