Behavior and Ecology 0 the Asiatic Elephant in Southeastern Ceylon A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHAPTER 2 REVIEW of the LITERATURE 2.1 Taxa And
CHAPTER 2 REVIEW OF THE LITERATURE 2.1 Taxa and Classification of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.11 Taxa and Classification of Acalypha indica Linn. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Euphorbiales Family : Euphorbiaceae Subfamily : Acalyphoideae Genus : Acalypha Species : Acalypha indica Linn. (Saha and Ahmed, 2011) Plant Synonyms: Acalypha ciliata Wall., A. canescens Wall., A. spicata Forsk. (35) Common names: Brennkraut (German), alcalifa (Brazil) and Ricinela (Spanish) (36). 9 2.12 Taxa and Classification of Bridelia retusa (L.) A. Juss. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Bridelia Species : Bridelia retusa (L.) A. Juss. Plant Synonyms: Bridelia airy-shawii Li. Common names: Ekdania (37,38). 2.13 Taxa and Classification of Cleidion javanicum BL. Kingdom : Plantae Subkingdom : Tracheobionta Superdivision : Spermatophyta Division : Magnoliophyta Class : Magnoliopsida Subclass : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Cleidion Species : Cleidion javanicum BL. Plant Synonyms: Acalypha spiciflora Burm. f. , Lasiostylis salicifolia Presl. Cleidion spiciflorum (Burm.f.) Merr. Common names: Malayalam and Yellari (39). 10 2.2 Review of chemical composition and bioactivities of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.2.1 Review of chemical composition and bioactivities of Acalypha indica Linn. Acalypha indica -

UNDERSTANDING HORSE BEHAVIOR Prepared By: Warren Gill, Professor Doyle G
4-H MEMBER GUIDE Agricultural Extension Service Institute of Agriculture HORSE PROJECT PB1654 UNIT 8 GRADE 12 UUNDERSTANDINGNDERSTANDING HHORSEORSE BBEHAVIOREHAVIOR 1 CONTENTS Introduction 3 Planning Your Project 3 The Basics of Horse Behavior 3 Types of Behavior 4 Horse Senses 4 Horse Communication 10 Domestication & Behavior 11 Mating Behavior 11 Behavior at Foaling Time 13 Feeding Behavior 15 Abnormal Behavior / Vices 18 Questions and Answers about Horses 19 References 19 Exercises 20 Glossary 23 SKILLS AND KNOWLEDGE TO BE ACQUIRED • Improved understanding of why horses behave like horses • Applying basic behavioral knowledge to improve training skills • Learning to prevent and correct behavioral problems • Better ways to manage horses through better understanding of horse motivation OBJECTIVES To help you: • Be more competent in horse-related skills and knowledge • Feel more confident around horses • Understand the applications of basic knowledge to practical problems REQUIREMENTS 1. Make a project plan 2. Complete this manual 3. Work on this project with others, including other 4-H members, 4-H leaders, your 4-H agent and other youth and adults who can assist you in your project. 4. Evaluate your accomplishments cover photo by2 Lindsay German UNDERSTANDING HORSE BEHAVIOR Prepared by: Warren Gill, Professor Doyle G. Meadows, Professor James B. Neel, Professor Animal Science Department The University of Tennessee INTRODUCTION he 4-H Horse Project offers 4-H’ers opportunities for growing and developing interest in horses. This manual should help expand your knowledge about horse behavior, which will help you better under T stand why a horse does what it does. The manual contains information about the basics of horse behavior, horse senses, domestication, mating behavior, ingestive (eating) behavior, foaling-time behavior and how horses learn. -

Environmental Assessment DOI-BLM-ORWA-B050-2018-0016-EA
United States Department of the Interior Bureau of Land Management Burns District Office 28910 Highway 20 West Hines, Oregon 97738 541-589-4400 Phone 541-573-4411 Fax Spay Feasibility and On-Range Behavioral Outcomes Assessment and Warm Springs HMA Population Management Plan Environmental Assessment DOI-BLM-ORWA-B050-2018-0016-EA June 29, 2018 This Page is Intentionally Left Blank Spay Feasibility and On-Range Behavioral Outcomes Assessment and Warm Springs HMA Population Management Plan Environmental Assessment DOI-BLM-ORWA-B050-2018-0016-EA Table of Contents I. INTRODUCTION .........................................................................................................1 A. Background................................................................................................................ 1 B. Purpose and Need for Proposed Action..................................................................... 4 C. Decision to be Made .................................................................................................. 5 D. Conformance with BLM Resource Management Plan(s) .......................................... 6 E. Consistency with Laws, Regulations and Policies..................................................... 7 F. Scoping and Identification of Issues ........................................................................ 12 1. Issues for Analysis .......................................................................................... 13 2. Issues Considered but Eliminated from Detailed Analysis ............................ -

Download 3578.Pdf
z Available online at http://www.journalajst.com INTERNATIONAL JOURNAL OF CURRENT RESEARCH International Journal of Current Research Vol. 5, Issue, 06, pp.1599-1602, June, 2013 ISSN: 0975-833X RESEARCH ARTICLE PHARMACOGNOSTIC EVALUATION OF Bridelia retusa SPRENG. Ranjan, R. and *Deokule, S. S. Department of Botany, University of Pune, Pune-411007 (M.S) India ARTICLE INFO ABSTRACT Article History: Bridelia retusa Spreng belong to family Euphorbiaceae and is being used in the indigenous systems of medicine for the treatment of rheumatism and also used as astringent. The drug part used is the grayish brown roots of this Received 12th March, 2013 plant. The species is also well known in ayurvedic medicine for kidney stone. The present paper reveals the Received in revised form botanical standardization on the root of B.retusa. The Pharmacognostic studies include macroscopic, microscopic 11th April, 2013 characters, histochemistry and phytochemistry. The phytochemical and histochemical test includes starch, protein, Accepted 13rd May, 2013 saponin, sugar, tannins, glycosides and alkaloids. Percentage extractives, ash and acid insoluble ash, fluorescence Published online 15th June, 2013 analysis and HPTLC. Key words: Bridelia retusa, Pharmacognostic standardization, Phytochemical analysis, HPTLC. Copyright, IJCR, 2013, Academic Journals. All rights reserved. INTRODUCTION Microscopic and Macroscopic evaluation Thin (25μ) hand cut sections were taken from the fresh roots, Bridelia retusa Spreng. belongs to family Euphorbiaceae is permanent double stained and finally mounted in Canada balsam as distributed in India, Srilanka, Myanmar, Thailand, Indochina, Malay per the plant micro techniques method of (Johansen, 1940).The Peninsula and Sumatra ( Anonymous, 1992) The plant body is erect macroscopic evaluation was studied by the following method of and a large deciduous tree. -

An Economic Analysis of Intersectoral Water Allocation In
AN ECONOMIC ANALYSIS OF INTERSECTORAL WATER ALLOCATION IN SOUTHEASTERN SRI LANKA BY SARATH PARAKRAMA WELIGAMAGE A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY School of Earth and Environmental Sciences AUGUST 2011 To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of SARATH PARAKRAMA WELIGAMAGE find it satisfactory and recommend that it be accepted. ___________________________________ Keith A. Blatner, Ph.D., Chair ___________________________________ C. Richard Shumway, Ph.D. ___________________________________ Jill J. McCluskey, Ph.D. ii ACKNOWLEDGMENT Earning a PhD from WSU fulfills a long held aspiration in my life to earn a doctorate from a US university. I thank all those who have contributed to my achieving this goal at Washington State University. Frank Rijsberman, Director General (2001-2007) of the International Water Management Institute (IWWI), was the key person behind meeting my aspiration to earn a PhD. His vision and passion for capacity building of scientific manpower in the South led to the initiation of the IWMI’s program for capacity building that supported my dissertation research. Frank also authorized the initial support for my PhD program. I thank Frank’s successor Colin Chartres, and David Molden, Interim Director General for continued support to me. At WSU, my major professor Keith Blatner was the key person behind fulfilling my goals. In addition to his unmatched knowledge spanning across many disciplines, Keith was a constant source of support and I also appreciate his compassion and empathy. I thank Richard Shumway for helping me fulfill my academic aspirations at a very high level. -

Pterospermum Acerifolium(L)
Human Journals Research Article January 2019 Vol.:14, Issue:2 © All rights are reserved by Bora Biswa Jyoti et al. A Pharmacognostic and Phyto-Physicochemical Evaluation of Pterospermum acerifolium (L) Wild. Flower Keywords: Pterospermum acerifolium, ethnobotanical uses, pharmacognosy, pharmacological activities, phytochemistry. ABSTRACT Bora Biswa Jyoti*, Kotoky Jibon1 Pterospermum acerifolium (L) Wild. is usually a perennial, evergreen tree belonging to family Sterculiaceae distributed *Dept. of DG, Govt. Ayurvedic College, Guwahati throughout the world. It is found in the sub-Himalayan tract, (Assam) – India outer Himalayan valleys, and hills up to 4,000 ft., Assam, West Bengal, Khasi Hills, Manipur, Darjeeling, Odisha and extensively planted in Maharashtra state. It is commonly known 1. Division of Life Sciences, Institute of Advanced as Bayur Tree, Dinner-plate tree, Kanak Champa or Study in Science & Technology, (Assam) India Muchukunda. It is one among widely used ethnomedicinal plants for various diseases in India. Various parts of this tree Submission: 21 December 2018 have been traditionally used for a number of disorders including Accepted: 26 December 2018 cancer. Mainly it is used for karna-shula (an earache), chechaka (smallpox), sweta-pradara (leucorrhoea), sotha (inflammation), Published: 30 January 2019 dust-vrana (ulcers), kustha (leprosy), prameha (diabetes syndrome). In view of growing popularity and global interest in Ayurveda and its drug lore. There is an imminent need for well- coordinated research touching phyto-physicochemical, pharmacological as well as clinical studies of plant drugs. It is especially necessary to satisfy the international bodies and drug www.ijppr.humanjournals.com regulatory authorities relating to standards and quality control of the drug used. -

Water Balance Variability Across Sri Lanka for Assessing Agricultural and Environmental Water Use W.G.M
Agricultural Water Management 58 (2003) 171±192 Water balance variability across Sri Lanka for assessing agricultural and environmental water use W.G.M. Bastiaanssena,*, L. Chandrapalab aInternational Water Management Institute (IWMI), P.O. Box 2075, Colombo, Sri Lanka bDepartment of Meteorology, 383 Bauddaloka Mawatha, Colombo 7, Sri Lanka Abstract This paper describes a new procedure for hydrological data collection and assessment of agricultural and environmental water use using public domain satellite data. The variability of the annual water balance for Sri Lanka is estimated using observed rainfall and remotely sensed actual evaporation rates at a 1 km grid resolution. The Surface Energy Balance Algorithm for Land (SEBAL) has been used to assess the actual evaporation and storage changes in the root zone on a 10- day basis. The water balance was closed with a runoff component and a remainder term. Evaporation and runoff estimates were veri®ed against ground measurements using scintillometry and gauge readings respectively. The annual water balance for each of the 103 river basins of Sri Lanka is presented. The remainder term appeared to be less than 10% of the rainfall, which implies that the water balance is suf®ciently understood for policy and decision making. Access to water balance data is necessary as input into water accounting procedures, which simply describe the water status in hydrological systems (e.g. nation wide, river basin, irrigation scheme). The results show that the irrigation sector uses not more than 7% of the net water in¯ow. The total agricultural water use and the environmental systems usage is 15 and 51%, respectively of the net water in¯ow. -

Environmental and Social Values of River Water: Examples from the Menik Ganga, Sri Lanka
Q:\2004 projects\IKG Services Unit Templates\IWMI Working Papers\WP Cover_with Specs A4.ai Spread size: A3 (420 x 297 mm)Paper size: A4 (297 x 210 inches) when spread is folded onceA 10p0 C WORKING PAPER 121 Environmental and Social A. Working paper numberNewsGoth BT, 18pt. Bold, in Values of River Water: 100% Black B. Country series no.NewsGoth BT Light, 14pt., 85% Examples from the Menik condensed, in 100% Black Ganga, Sri Lanka C. Paper titleNewsGoth BT 32pt. (can be smaller to be B 28pt. if the title is longer) on 42 (leading), 85% condensed, left aligned, in 100% Pantone 2935 4p0 D. Sub titleNewsGoth BT Condensed, 24pt. on 34 Priyanka Dissanayake and Vladimir Smakhtin (leading), left aligned, in 100% Black H E. Name(s) of authorsNewsGoth BT Roman, 12pt. on 16 (leading) 90% condensed, left aligned, top margin aligned with the top margin of the photograph, in 4p0 100% Black D F. Other logos (if any)Aligned with IWMI logo (bottom) G. IWMI and Future Harvest logosPlaced on the vertical band Postal Address: P O Box 2075 E Colombo Sri Lanka H. BackgroundPMS (Pantone) 134 C, bleed Location: 127, Sunil Mawatha 25p6 Max. text area Pelawatta Battaramulla 2p0 1p6 Sri Lanka Tel: +94-11 2880000 G Fax: +94-11 2786854 E-mail: [email protected] F Website: 2p0 http://www.iwmi.org SM International International Water Management IWMI isaFuture Harvest Center Water Management Institute supportedby the CGIAR ISBN: 978-92-9090-674-2 Institute 3p0 K PANTONE 2935 C PANTONE 134 C Working Paper 121 Environmental and Social Values of River Water: Examples from the Menik Ganga, Sri Lanka Priyanka Dissanayake and Vladimir Smakhtin International Water Management Institute IWMI receives its principal funding from 58 governments, private foundations, and international and regional organizations known as the Consultative Group on International Agricultural Research (CGIAR). -

Assessing the Environmental Adaptation of Wildlife And
Assessing the Environmental Adaptation of Wildlife and Production Animals Production and Wildlife of Adaptation Assessing Environmental the Assessing the Environmental Adaptation of Wildlife and • Edward Narayan Edward • Production Animals Applications of Physiological Indices and Welfare Assessment Tools Edited by Edward Narayan Printed Edition of the Special Issue Published in Animals www.mdpi.com/journal/animals Assessing the Environmental Adaptation of Wildlife and Production Animals: Applications of Physiological Indices and Welfare Assessment Tools Assessing the Environmental Adaptation of Wildlife and Production Animals: Applications of Physiological Indices and Welfare Assessment Tools Editor Edward Narayan MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade • Manchester • Tokyo • Cluj • Tianjin Editor Edward Narayan The University of Queensland Australia Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Animals (ISSN 2076-2615) (available at: https://www.mdpi.com/journal/animals/special issues/ environmental adaptation). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Volume Number, Page Range. ISBN 978-3-0365-0142-0 (Hbk) ISBN 978-3-0365-0143-7 (PDF) © 2021 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher areproperly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. -

Downloaded from Brill.Com10/07/2021 08:53:11AM Via Free Access 130 IAWA Journal, Vol
IAWA Journal, Vol. 27 (2), 2006: 129–136 WOOD ANATOMY OF CRAIGIA (MALVALES) FROM SOUTHEASTERN YUNNAN, CHINA Steven R. Manchester1, Zhiduan Chen2 and Zhekun Zhou3 SUMMARY Wood anatomy of Craigia W.W. Sm. & W.E. Evans (Malvaceae s.l.), a tree endemic to China and Vietnam, is described in order to provide new characters for assessing its affinities relative to other malvalean genera. Craigia has very low-density wood, with abundant diffuse-in-aggre- gate axial parenchyma and tile cells of the Pterospermum type in the multiseriate rays. Although Craigia is distinct from Tilia by the pres- ence of tile cells, they share the feature of helically thickened vessels – supportive of the sister group status suggested for these two genera by other morphological characters and preliminary molecular data. Although Craigia is well represented in the fossil record based on fruits, we were unable to locate fossil woods corresponding in anatomy to that of the extant genus. Key words: Craigia, Tilia, Malvaceae, wood anatomy, tile cells. INTRODUCTION The genus Craigia is endemic to eastern Asia today, with two species in southern China, one of which also extends into northern Vietnam and southeastern Tibet. The genus was initially placed in Sterculiaceae (Smith & Evans 1921; Hsue 1975), then Tiliaceae (Ren 1989; Ying et al. 1993), and more recently in the broadly circumscribed Malvaceae s.l. (including Sterculiaceae, Tiliaceae, and Bombacaceae) (Judd & Manchester 1997; Alverson et al. 1999; Kubitzki & Bayer 2003). Similarities in pollen morphology and staminodes (Judd & Manchester 1997), and chloroplast gene sequence data (Alverson et al. 1999) have suggested a sister relationship to Tilia. -
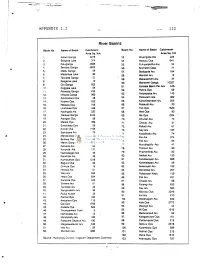
River Basins
APPENDIX I.I 122 River Basins Basin No Name of Basin Catchment Basin No. Name of Basin Catchment Area Sq. Km. Area Sq. Km 1. Kelani Ganga 2278 53. Miyangolla Ela 225 2. Bolgoda Lake 374 54. Maduru Oya 1541 3. Kaluganga 2688 55. Pulliyanpotha Aru 52 4. Bemota Ganga 6622 56. Kirimechi Odai 77 5. Madu Ganga 59 57. Bodigoda Aru 164 6. Madampe Lake 90 58. Mandan Aru 13 7. Telwatte Ganga 51 59. Makarachchi Aru 37 8. Ratgama Lake 10 60. Mahaweli Ganga 10327 9. Gin Ganga 922 61. Kantalai Basin Per Ara 445- 10. Koggala Lake 64 62. Panna Oya 69 11. Polwatta Ganga 233 12. Nilwala Ganga 960 63. Palampotta Aru 143 13. Sinimodara Oya 38 64. Pankulam Ara 382 14. Kirama Oya 223 65. Kanchikamban Aru 205 15. Rekawa Oya 755 66. Palakutti A/u 20 16. Uruhokke Oya 348 67. Yan Oya 1520 17. Kachigala Ara 220 68. Mee Oya 90 18. Walawe Ganga 2442 69. Ma Oya 1024 19. Karagan Oya 58 70. Churian A/u 74 20. Malala Oya 399 71. Chavar Aru 31 21. Embilikala Oya 59 72. Palladi Aru 61 22. Kirindi Oya 1165 73. Nay Ara 187 23. Bambawe Ara 79 74. Kodalikallu Aru 74 24. Mahasilawa Oya 13 75. Per Ara 374 25. Butawa Oya 38 76. Pali Aru 84 26. Menik Ganga 1272 27. Katupila Aru 86 77. Muruthapilly Aru 41 28. Kuranda Ara 131 78. Thoravi! Aru 90 29. Namadagas Ara 46 79. Piramenthal Aru 82 30. Karambe Ara 46 80. Nethali Aru 120 31. -

Proceedings of the First Young Water Professionals Symposium
Proceedings of the First Young Water Professionals Symposium 22nd and 23rd November 2012 Galadari Hotel, Colombo Organized by Sri Lanka Water Partnership (Lanka Jalani) In association with International Water Management Institute (IWMI) and Unilever-Pureit i ISBN 978-955-4784-00-0 ii Table of Contents Page Abbreviations iv Foreword v Symposium Organization vi Report on Proceedings 1 Papers presented at Technical Sessions 12 Papers accepted but not presented 175 Annexes 1) Technical Sessions - Themes and aspects covered 215 2) Technical Sessions - Programme Agenda 216 3) Technical Sessions -Presentations - Summary of Discussion 219 4) List of Participants 227 iii Abbreviations CBO - Community Based Organization CKD-U - Chronic Kidney Diseases, Unknown COD - Chemical Oxygen Demand IPCC - Intergovernmental Panel on Climate Change IWMI -International Water Management Institute IWRM -Integrated Water Resources Management NGOs - Non Governmental Organizations NSF - National Science Foundation O&M - Operations and Maintenance PAC - Powdered Activated Carbon R & D - Research and Development SLWP - Sri Lanka Water Partnership SPI - Standard Precipitation Index SWARM - Sustainable Water Resources Management UDDT - Urine Diversion Dry Toilet YWPS - Young Water Professionals Symposium iv Foreword The Young Water Professionals Symposium (YWPS) was an outcome of the efforts of the Sri Lanka Water Partnership (SLWP) Programme Committee which in early 2012 had identified the limited opportunities available to young water professionals to contribute to water sector issues as a constraint to the development of the sector. The YWPS was planned as a platform where these mid- career water professionals could make their voices heard and present innovative solutions that could be adopted to better plan and manage water resources in Sri Lanka.