Quantities, Units and Symbols in Physical Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Physical Chemistry
INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY Physical and Biophysical Chemistry Division IUPAC Vc^llclllul LICb . U 111 Lb cillCl Oy 111 DOlo in Physical Chemistry Third Edition Prepared for publication by E. Richard Cohen Tomislav Cvitas Jeremy G. Frey BcTt.il Holmstrom Kozo Kuchitsu R,oberto Marquardt Ian Mills Franco Pavese Martin Quack Jiirgen Stohner Herbert L. Strauss Michio Takanii Anders J Thor The first and second editions were prepared for publication by Ian Mills Tomislav Cvitas Klaus Homann Nikola Kallay Kozo Kuchitsu IUPAC 2007 RSC Publishing CONTENTS v PREFACE ix HISTORICAL INTRODUCTION xi 1 PHYSICAL QUANTITIES AND UNITS 1 1.1 Physical quantities and quantity calculus 3 1.2 Base quantities and derived quantities 4 1.3 Symbols for physical quantities and units 5 1.3.1 General rules for symbols for quantities 5 1.3.2 General rules for symbols for units 5 1.4 Use of the words "extensive", "intensive", "specific", and "molar" 6 1.5 Products and quotients of physical quantities and units 7 1.6 The use of italic and Roman fonts for symbols in scientific publications 7 2 TABLES OF PHYSICAL QUANTITIES 11 2.1 Space and time 13 2.2 Classical mechanics 14 2.3 Electricity and magnetism 16 2.4 Quantum mechanics and quantum chemistry 18 2.4.1 Ab initio Hartree-Fock self-consistent field theory (ab initio SCF) 20 2.4.2 Hartree-Fock-Roothaan SCF theory, using molecular orbitals expanded as linear combinations of atomic-orbital basis . functions (LCAO-MO theory) . 21 2.5 Atoms and molecules 22 2.6 Spectroscopy 25 2.6.1 Symbols for angular -

A Learner's Guide to SI Units and Their Conversion
A Learner's Guide to SI Units and their Conversion October 2004 Workbook for students Learner's Guide to SI Units and their Conversion their and Units SI to Guide Learner's Edexcel A London Qualifications is one of the leading examining and awarding bodies in the UK and throughout the world. It incorporates all the qualifications previously awarded under the Edexcel and BTEC brand. We provide a wide range of qualifications including general (academic), vocational, occupational and specific programmes for employers. Through a network of UK and overseas offices, our centres receive the support they need to help them deliver their education and training programmes to learners. For further information please call Customer Services on 0870 240 9800, or visit our website at www.edexcel.org.uk Authorised by Jim Dobson Prepared by Sarah Harrison All the material in this publication is copyright © London Qualifications Limited 2004 CONTENTS Introduction 1 What are units? 2 Operations with units 5 Submultiple and multiple units 9 Conversion of units 11 Conversion examples and exercises 13 Length 13 Area 14 Volume 15 Mass 16 Time 17 Temperature 18 Density 19 Force 20 Stress and pressure 21 Answers to exercises 23 Introduction One of the important areas where Science and Technology students need support is in the conversion of units. This booklet is designed to be useful for students in all Science, Technology and Engineering subjects. This booklet has been produced to: S introduce students to SI base and derived units and S help students with the conversion of multiple and sub-multiple units to SI base and derived units. -

Far Infrared Radiation Exposure
INTERNATIONAL COMMISSION ON NON‐IONIZING RADIATION PROTECTION ICNIRP STATEMENT ON FAR INFRARED RADIATION EXPOSURE PUBLISHED IN: HEALTH PHYSICS 91(6):630‐645; 2006 ICNIRP PUBLICATION – 2006 ICNIRP Statement ICNIRP STATEMENT ON FAR INFRARED RADIATION EXPOSURE The International Commission on Non-Ionizing Radiation Protection* INTRODUCTION the health hazards associated with these hot environ- ments. Heat strain and discomfort (thermal pain) nor- THE INTERNATIONAL Commission on Non Ionizing Radia- mally limit skin exposure to infrared radiation levels tion Protection (ICNIRP) currently provides guidelines below the threshold for skin-thermal injury, and this is to limit human exposure to intense, broadband infrared particularly true for sources that emit largely IR-C. radiation (ICNIRP 1997). The guidelines that pertained Furthermore, limits for lengthy infrared exposures would to infrared radiation (IR) were developed initially with an have to consider ambient temperatures. For example, an aim to provide guidance for protecting against hazards infrared irradiance of 1 kW mϪ2 (100 mW cmϪ2)atan from high-intensity artificial sources and to protect work- ambient temperature of 5°C can be comfortably warm- ers in hot industries. Detailed guidance for exposure to ing, but at an ambient temperature of 30°C this irradiance longer far-infrared wavelengths (referred to as IR-C would be painful and produce severe heat strain. There- radiation) was not provided because the energy at longer fore, ICNIRP provided guidelines to limit skin exposure wavelengths from most lamps and industrial infrared to pulsed sources and very brief exposures where thermal sources of concern actually contribute only a small injury could take place faster than the pain response time fraction of the total radiant heat energy and did not and where environmental temperature and the irradiated require measurement. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

2019 Redefinition of SI Base Units
2019 redefinition of SI base units A redefinition of SI base units is scheduled to come into force on 20 May 2019.[1][2] The kilogram, ampere, kelvin, and mole will then be defined by setting exact numerical values for the Planck constant (h), the elementary electric charge (e), the Boltzmann constant (k), and the Avogadro constant (NA), respectively. The metre and candela are already defined by physical constants, subject to correction to their present definitions. The new definitions aim to improve the SI without changing the size of any units, thus ensuring continuity with existing measurements.[3][4] In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes,[5][6] which the International Committee for Weights and Measures (CIPM) had proposed earlier that year.[7]:23 The previous major change of the metric system was in 1960 when the International System of Units (SI) was formally published. The SI is a coherent system structured around seven base units whose definitions are unconstrained by that of any other unit and another twenty-two named units derived from these base units. The metre was redefined in terms of the wavelength of a spectral line of a The SI system after the 2019 redefinition: krypton-86 radiation,[Note 1] making it derivable from universal natural Dependence of base unit definitions onphysical constants with fixed numerical values and on other phenomena, but the kilogram remained defined in terms of a physical prototype, base units. leaving it the only artefact upon which the SI unit definitions depend. The metric system was originally conceived as a system of measurement that was derivable from unchanging phenomena,[8] but practical limitations necessitated the use of artefacts (the prototype metre and prototype kilogram) when the metric system was first introduced in France in 1799. -
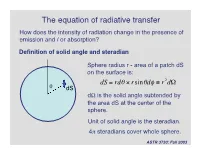
The Equation of Radiative Transfer How Does the Intensity of Radiation Change in the Presence of Emission and / Or Absorption?
The equation of radiative transfer How does the intensity of radiation change in the presence of emission and / or absorption? Definition of solid angle and steradian Sphere radius r - area of a patch dS on the surface is: dS = rdq ¥ rsinqdf ≡ r2dW q dS dW is the solid angle subtended by the area dS at the center of the † sphere. Unit of solid angle is the steradian. 4p steradians cover whole sphere. ASTR 3730: Fall 2003 Definition of the specific intensity Construct an area dA normal to a light ray, and consider all the rays that pass through dA whose directions lie within a small solid angle dW. Solid angle dW dA The amount of energy passing through dA and into dW in time dt in frequency range dn is: dE = In dAdtdndW Specific intensity of the radiation. † ASTR 3730: Fall 2003 Compare with definition of the flux: specific intensity is very similar except it depends upon direction and frequency as well as location. Units of specific intensity are: erg s-1 cm-2 Hz-1 steradian-1 Same as Fn Another, more intuitive name for the specific intensity is brightness. ASTR 3730: Fall 2003 Simple relation between the flux and the specific intensity: Consider a small area dA, with light rays passing through it at all angles to the normal to the surface n: n o In If q = 90 , then light rays in that direction contribute zero net flux through area dA. q For rays at angle q, foreshortening reduces the effective area by a factor of cos(q). -

Relationships of the SI Derived Units with Special Names and Symbols and the SI Base Units
Relationships of the SI derived units with special names and symbols and the SI base units Derived units SI BASE UNITS without special SI DERIVED UNITS WITH SPECIAL NAMES AND SYMBOLS names Solid lines indicate multiplication, broken lines indicate division kilogram kg newton (kg·m/s2) pascal (N/m2) gray (J/kg) sievert (J/kg) 3 N Pa Gy Sv MASS m FORCE PRESSURE, ABSORBED DOSE VOLUME STRESS DOSE EQUIVALENT meter m 2 m joule (N·m) watt (J/s) becquerel (1/s) hertz (1/s) LENGTH J W Bq Hz AREA ENERGY, WORK, POWER, ACTIVITY FREQUENCY second QUANTITY OF HEAT HEAT FLOW RATE (OF A RADIONUCLIDE) s m/s TIME VELOCITY katal (mol/s) weber (V·s) henry (Wb/A) tesla (Wb/m2) kat Wb H T 2 mole m/s CATALYTIC MAGNETIC INDUCTANCE MAGNETIC mol ACTIVITY FLUX FLUX DENSITY ACCELERATION AMOUNT OF SUBSTANCE coulomb (A·s) volt (W/A) C V ampere A ELECTRIC POTENTIAL, CHARGE ELECTROMOTIVE ELECTRIC CURRENT FORCE degree (K) farad (C/V) ohm (V/A) siemens (1/W) kelvin Celsius °C F W S K CELSIUS CAPACITANCE RESISTANCE CONDUCTANCE THERMODYNAMIC TEMPERATURE TEMPERATURE t/°C = T /K – 273.15 candela 2 steradian radian cd lux (lm/m ) lumen (cd·sr) 2 2 (m/m = 1) lx lm sr (m /m = 1) rad LUMINOUS INTENSITY ILLUMINANCE LUMINOUS SOLID ANGLE PLANE ANGLE FLUX The diagram above shows graphically how the 22 SI derived units with special names and symbols are related to the seven SI base units. In the first column, the symbols of the SI base units are shown in rectangles, with the name of the unit shown toward the upper left of the rectangle and the name of the associated base quantity shown in italic type below the rectangle. -

International System of Units (SI)
International System of Units (SI) National Institute of Standards and Technology (www.nist.gov) Multiplication Prefix Symbol factor 1018 exa E 1015 peta P 1012 tera T 109 giga G 106 mega M 103 kilo k 102 * hecto h 101 * deka da 10-1 * deci d 10-2 * centi c 10-3 milli m 10-6 micro µ 10-9 nano n 10-12 pico p 10-15 femto f 10-18 atto a * Prefixes of 100, 10, 0.1, and 0.01 are not formal SI units From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Seven basic SI units Physical quantity Unit Symbol Length meter m Mass kilogram kg Time second s Electrical current ampere A Thermodynamic temperature kelvin K Amount of substance mole mol Luminous intensity candela cd Supplementary SI units Physical quantity Unit Symbol Plane angle radian rad Solid angle steradian sr From: Downs, R.J. 1988. HortScience 23:881-812. International System of Units (SI) Derived SI units with special names Physical quantity Unit Symbol Derivation Absorbed dose gray Gy J kg-1 Capacitance farad F A s V-1 Conductance siemens S A V-1 Disintegration rate becquerel Bq l s-1 Electrical charge coulomb C A s Electrical potential volt V W A-1 Energy joule J N m Force newton N kg m s-2 Illumination lux lx lm m-2 Inductance henry H V s A-1 Luminous flux lumen lm cd sr Magnetic flux weber Wb V s Magnetic flux density tesla T Wb m-2 Pressure pascal Pa N m-2 Power watt W J s-1 Resistance ohm Ω V A-1 Volume liter L dm3 From: Downs, R.J. -
![Homework 1: Exercise 1 Metric - English Conversion [Based on the Chauffe & Jefferies (2007)]](https://docslib.b-cdn.net/cover/5363/homework-1-exercise-1-metric-english-conversion-based-on-the-chauffe-jefferies-2007-975363.webp)
Homework 1: Exercise 1 Metric - English Conversion [Based on the Chauffe & Jefferies (2007)]
MAR 110 HW 1: Exercise 1 – Conversions p. 1 Homework 1: Exercise 1 Metric - English Conversion [based on the Chauffe & Jefferies (2007)] 1-1. THE METRIC SYSTEM The French developed the metric system during the 1790s. Unlike the "English" system, in which the difference between smaller and larger units is irregular and has no scientific foundation, the metric system is based on multiples of 10. Smaller and larger units can be obtained simply by moving the decimal point - that is multiplying or dividing by 10. For example, in the "English" system there are 12 inches to a foot (ft), but 3 ft to a yard, and 1760 yards in a mile. By contrast, in the metric system there are 10 m to a decameter (dm), 10 dm to a hectometer (hm), and 10 hm to a kilometer (km). The convenience and regularity of the metric system make it the standard system used in scientific research The basic units of the metric system are the meter for length, gram for mass (weight), liter for volume, and degree Celsius (OC) for temperature. These terms have been defined in terms of practical considerations. For example, one meter is equal to one ten millionth of the distance between the North Pole and the Equator. The gram is the mass of one cubic centimeter (or one millionth of a cubic meter) of water at a temperature of 4°C. The liter is the volume of a cubic decimeter (or one thousandth of a cubic meter). A degree Celsius is one-hundredth of the change in temperature between the freezing and boiling points of water. -

Glossary of Terms
GLOSSARY OF TERMS Terminology Used for Ultraviolet (UV) Curing Process Design and Measurement This glossary of terms has been assembled in order to provide users, formulators, suppliers and researchers with terms that are used in the design and measurement of UV curing systems. It was prompted by the scattered and sometimes incorrect terms used in industrial UV curing technologies. It is intended to provide common and technical meanings as used in and appropriate for UV process design, measurement, and specification. General scientific terms are included only where they relate to UV Measurements. The object is to be "user-friendly," with descriptions and comments on meaning and usage, and minimum use of mathematical and strict definitions, but technically correct. Occasionally, where two or more terms are used similarly, notes will indicate the preferred term. For historical and other reasons, terms applicable to UV Curing may vary slightly in their usage from other sciences. This glossary is intended to 'close the gap' in technical language, and is recommended for authors, suppliers and designers in UV Curing technologies. absorbance An index of the light or UV absorbed by a medium compared to the light transmitted through it. Numerically, it is the logarithm of the ratio of incident spectral irradiance to the transmitted spectral irradiance. It is unitless number. Absorbance implies monochromatic radiation, although it is sometimes used as an average applied over a specified wavelength range. absorptivity (absorption coefficient) Absorbance per unit thickness of a medium. actinometer A chemical system or physical device that determines the number of photons in a beam integrally or per unit time. -

Princeton University Laser Safety Training Guide
Laser Safety Training Guide Environmental Health and Safety http://www.princeton.edu/ehs September 2007 LASER SAFETY TRAINING GUIDE TABLE OF CONTENTS SECTION 1: LASER FUNDAMENTALS 3 LASER Theory And Operation 4 Components Of A Laser 5 Types Of Lasers 6 SECTION 2: LASER HAZARDS 8 BEAM-RELATED HAZARDS 8 Types of Beam Exposure 9 Eye 9 Skin 12 NON-BEAM HAZARDS 12 Electrical Hazards 13 Laser Generated Air Contaminants -- The “Plume” 14 Collateral and Plasma Radiation 14 Fire Hazards 15 Compressed Gases 15 Laser Dyes 15 SECTION 3: LASER HAZARD CLASSIFICATION 17 SECTION 4: LASER CONTROL MEASURES 19 Maximum Permissible Exposure (MPE) 19 Accessible Exposrue Limit 19 Optical Density (OD) 19 Nominal Hazard Zone (NHZ) 20 Control Measures by Laser Classification 21 Protective Equipment 27 Protective Eyewear 27 Laser Eye Protection Selection Process 27 Other Protective Equipment 28 Special Controls for UltraViolet and Infrared Lasers 29 SECTION 5: LASER SAFETY AT PRINCETON UNIVERSITY 30 SECTION 6: GLOSSARY 33 APPENDIX A: SELECTED ANSI STANDARD TABLES 36 APPENDIX B: LASER SAFETY TIPS 43 APPENDIX C: LASER SAFETY CHECKLIST 44 2 Section 1: LASER FUNDAMENTALS Introduction The word laser is an acronym for Light Amplification by Stimulated Emission of Radiation. Lasers are used as research aides in many departments at Princeton University. In this document, the word laser will be limited to electromagnetic radiation-emitting devices using light amplification by stimulated emission of radiation at wavelengths from 180 nanometers to 1 millimeter. The electromagnetic spectrum includes energy ranging from gamma rays to electricity. Figure 1 illustrates the total electromagnetic spectrum and wavelengths of the various regions. -
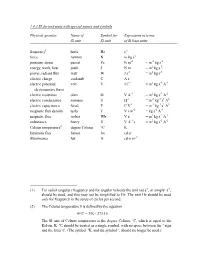
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K.