BALMNH No 29 2012.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nonnative Reptilies in South Florida ID Guide
Nonnative Reptiles in South Florida Identification Guide • The nonnative reptiles shown here are native to Central and South America, Asia, and Nonnative species are Africa. They were introduced to south Florida by human activity. sometimes confused with • Invasive species harm native species through direct predation, competition for resources, the Florida natives shown spread of disease, and disruption of natural ecosystems. Many of the nonnative reptiles on because their colorations this guide are, or have the potential to become, invasive. and patterns are very • Use this guide to identify invasive species and immediately report sightings of the black similar. Pay attention to the and white tegu, Nile monitor, and all invasive snakes to 1-888-IVE-GOT1. Take a distinct characteristics and photo and note the location relative to street intersections or with a GPS if possible. typical adult sizes listed on this guide to avoid • More photos can be found at www.flmnh.ufl.edu/herpetology/herpetology.htm. confusion when you • Be certain that an animal is a nonnative species before removing it. Warning-most encounter these animals. reptiles will bite or scratch if provoked. Nonnative Lizards NATIVE :- • ,,.., •· t ..... Look-a-Likes . ... ·-tt-..... • •. .. l . 1 '\..\ =- ' . ----.....·~·-· - - ',-<•'-' ' . \:,' . <! •.t'- . ,. '\. Dav id 13,irbsv ~ ·- ~ 9111'.', o:'"' w:' Black and White Tegu 2 to 3 ft. Dark bands with plentiful white dots between them Eastern Fence Lizard 3.5 to 7.5 in. Northern Curly-Tailed Lizard 7 to 10.5 in . Gray to tan with curled tail Florida Scrub Lizard 3.5 to 5.5 in. American Alligator 6 to 9 ft. Nile Monitor 4 to 6 ft. -

Indiana Snakes Are Listed Here, and Not All Streams, Ponds and Lakes Suns Beside Creeks
Midwest Worm Snake Carphophis amoenus satiny gray below with a brown or dark amber Fox Snake Elaphe vulpina This snake version of the earthworm is iris of the eye. The blue racer may show Blue This snake of marshes and wet places has brown above and has a pink belly and sides. It varying shades of gunmetal gray or blue above bold blotches, a grayish- or brownish-yellow is secretive and seldom seen, spending most of and below with a darker head and eye area. Mixed body and a dull orange/reddish head and tail. its time under stones, boards and logs where Racers move fast and sometimes appear to Black It vibrates its tail if cornered, but rarely bites. the ground is moist. It feeds on soft-bodied “chase” people. In fact, this behavior is often Snakes insects and earthworms. associated with courtship and may be used to drive an Black Kingsnake Lampropeltis getulus getulus intruder out of a territory. This glossy black snake has speckles of Rough Green Snake Opheodrys aestivas white and cream that may be less apparent in Smooth Green Snake Opheodrys vernalis Eastern Milk Snake older snakes. It lives on streambanks and in Both species are green above with white, Lampropeltis triangulatum triangulatum moist meadows, where it feeds on other yellow or pale green bellies. The rough green Red Milk Snake snakes, turtle eggs, mice and voles. It is snake has keeled scales that give it a rough Lampropeltis triangulatum syspila “V” generally secretive and can be found under texture. This snake, listed as a species of This snake’s taste for mice makes pattern boards, logs and debris. -

Ornate Chorus Frog
Ornate Chorus Frog Pseudacris ornata Taxa: Amphibian SE-GAP Spp Code: aOCFR Order: Anura ITIS Species Code: 173531 Family: Hylidae NatureServe Element Code: AAABC05050 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_aOCFR.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_aOCFR.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=aOCFR Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/aOCFR_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: MS (Non-game species in need of management), NC (SR) NS Global Rank: G5 NS State Rank: AL (S5), FL (SNR), GA (S5), LA (S1), MS (S1), NC (S3), SC (SNR) aOCFR Page 1 of 4 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 14,156.0 < 1 2,389.3 < 1 0.0 0 0.0 0 Status 2 32,041.4 < 1 24,919.3 < 1 0.0 0 0.0 0 Status 3 3.2 < 1 181,436.9 4 0.0 0 75,398.1 2 Status 4 5.2 < 1 0.0 0 0.0 0 0.0 0 Total 46,205.9 < 1 208,745.5 4 0.0 0 75,398.1 2 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 7,947.2 < 1 8.6 < 1 706.9 < 1 Status 2 0.0 0 1,356.0 < 1 1,336.1 < 1 0.0 0 Status 3 13,565.2 < 1 84.2 < 1 0.0 0 1,051.8 < 1 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 13,565.2 < 1 9,387.5 < 1 1,344.6 < 1 1,758.7 < 1 Native Am. -

Land Areas of the National Forest System, As of September 30, 2019
United States Department of Agriculture Land Areas of the National Forest System As of September 30, 2019 Forest Service WO Lands FS-383 November 2019 Metric Equivalents When you know: Multiply by: To fnd: Inches (in) 2.54 Centimeters Feet (ft) 0.305 Meters Miles (mi) 1.609 Kilometers Acres (ac) 0.405 Hectares Square feet (ft2) 0.0929 Square meters Yards (yd) 0.914 Meters Square miles (mi2) 2.59 Square kilometers Pounds (lb) 0.454 Kilograms United States Department of Agriculture Forest Service Land Areas of the WO, Lands National Forest FS-383 System November 2019 As of September 30, 2019 Published by: USDA Forest Service 1400 Independence Ave., SW Washington, DC 20250-0003 Website: https://www.fs.fed.us/land/staff/lar-index.shtml Cover Photo: Mt. Hood, Mt. Hood National Forest, Oregon Courtesy of: Susan Ruzicka USDA Forest Service WO Lands and Realty Management Statistics are current as of: 10/17/2019 The National Forest System (NFS) is comprised of: 154 National Forests 58 Purchase Units 20 National Grasslands 7 Land Utilization Projects 17 Research and Experimental Areas 28 Other Areas NFS lands are found in 43 States as well as Puerto Rico and the Virgin Islands. TOTAL NFS ACRES = 192,994,068 NFS lands are organized into: 9 Forest Service Regions 112 Administrative Forest or Forest-level units 503 Ranger District or District-level units The Forest Service administers 149 Wild and Scenic Rivers in 23 States and 456 National Wilderness Areas in 39 States. The Forest Service also administers several other types of nationally designated -

Contributions of Intensively Managed Forests to the Sustainability of Wildlife Communities in the South
CONTRIBUTIONS OF INTENSIVELY MANAGED FORESTS TO THE SUSTAINABILITY OF WILDLIFE COMMUNITIES IN THE SOUTH T. Bently Wigley1, William M. Baughman, Michael E. Dorcas, John A. Gerwin, J. Whitfield Gibbons, David C. Guynn, Jr., Richard A. Lancia, Yale A. Leiden, Michael S. Mitchell, Kevin R. Russell ABSTRACT Wildlife communities in the South are increasingly influenced by land use changes associated with human population growth and changes in forest management strategies on both public and private lands. Management of industry-owned landscapes typically results in a diverse mixture of habitat types and spatial arrangements that simultaneously offers opportunities to maintain forest cover, address concerns about fragmentation, and provide habitats for a variety of wildlife species. We report here on several recent studies of breeding bird and herpetofaunal communities in industry-managed landscapes in South Carolina. Study landscapes included the 8,100-ha GilesBay/Woodbury Tract, owned and managed by International Paper Company, and 62,363-ha of the Ashley and Edisto Districts, owned and managed by Westvaco Corporation. Breeding birds were sampled in both landscapes from 1995-1999 using point counts, mist netting, nest searching, and territory mapping. A broad survey of herpetofauna was conducted during 1996-1998 across the Giles Bay/Woodbury Tract using a variety of methods, including: searches of natural cover objects, time-constrained searches, drift fences with pitfall traps, coverboards, automated recording systems, minnow traps, and turtle traps. Herpetofaunal communities were sampled more intensively in both landscapes during 1997-1999 in isolated wetland and selected structural classes. The study landscapes supported approximately 70 bird and 72 herpetofaunal species, some of which are of conservation concern. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

Hybridization Between Multiple Fence Lizard Lineages in an Ecotone
Molecular Ecology (2007) 16, 1035–1054 doi: 10.1111/j.1365-294X.2006.03194.x HybridizationBlackwell Publishing Ltd between multiple fence lizard lineages in an ecotone: locally discordant variation in mitochondrial DNA, chromosomes, and morphology ADAM D. LEACHÉ* and CHARLES J. COLE†‡ *Museum of Vertebrate Zoology and Department of Integrative Biology, 3101 Valley Life Sciences Building, University of California, Berkeley, CA 94720-3160, USA, †Department of Herpetology, American Museum of Natural History, New York, NY 10024-5192, USA Abstract We investigated a hybrid zone between two major lineages of fence lizards (Sceloporus cowlesi and Sceloporus tristichus) in the Sceloporus undulatus species complex in eastern Arizona. This zone occurs in an ecotone between Great Basin Grassland and Conifer Wood- land habitats. We analysed spatial variation in mtDNA (N = 401; 969 bp), chromosomes (N = 217), and morphology (N = 312; 11 characters) to characterize the hybrid zone and assess species limits. A fine-scale population level phylogenetic analysis refined the boundaries between these species and indicated that four nonsister mtDNA clades (three belonging to S. tristichus and one to S. cowlesi) are sympatric at the centre of the zone. Esti- mates of cytonuclear disequilibria in the population closest to the centre of the hybrid zone suggest that the S. tristichus clades are randomly mating, but that the S. cowlesi haplotype has a significant nonrandom association with nuclear alleles. Maximum-likelihood cline- fitting analyses suggest that the karyotype, morphology, and dorsal colour pattern clines are all coincident, but the mtDNA cline is skewed significantly to the south. A temporal comparison of cline centres utilizing karyotype data collected in the early 1970s and in 2002 suggests that the cline may have shifted by approximately 1.5 km to the north over a 30-year period. -

Checklist of Reptiles and Amphibians Revoct2017
CHECKLIST of AMPHIBIANS and REPTILES of ARCHBOLD BIOLOGICAL STATION, the RESERVE, and BUCK ISLAND RANCH, Highlands County, Florida. Voucher specimens of species recorded from the Station are deposited in the Station reference collections and the herpetology collection of the American Museum of Natural History. Occurrence3 Scientific name1 Common name Status2 Exotic Station Reserve Ranch AMPHIBIANS Order Anura Family Bufonidae Anaxyrus quercicus Oak Toad X X X Anaxyrus terrestris Southern Toad X X X Rhinella marina Cane Toad ■ X Family Hylidae Acris gryllus dorsalis Florida Cricket Frog X X X Hyla cinerea Green Treefrog X X X Hyla femoralis Pine Woods Treefrog X X X Hyla gratiosa Barking Treefrog X X X Hyla squirella Squirrel Treefrog X X X Osteopilus septentrionalis Cuban Treefrog ■ X X Pseudacris nigrita Southern Chorus Frog X X Pseudacris ocularis Little Grass Frog X X X Family Leptodactylidae Eleutherodactylus planirostris Greenhouse Frog ■ X X X Family Microhylidae Gastrophryne carolinensis Eastern Narrow-mouthed Toad X X X Family Ranidae Lithobates capito Gopher Frog X X X Lithobates catesbeianus American Bullfrog ? 4 X X Lithobates grylio Pig Frog X X X Lithobates sphenocephalus sphenocephalus Florida Leopard Frog X X X Order Caudata Family Amphiumidae Amphiuma means Two-toed Amphiuma X X X Family Plethodontidae Eurycea quadridigitata Dwarf Salamander X Family Salamandridae Notophthalmus viridescens piaropicola Peninsula Newt X X Family Sirenidae Pseudobranchus axanthus axanthus Narrow-striped Dwarf Siren X Pseudobranchus striatus -
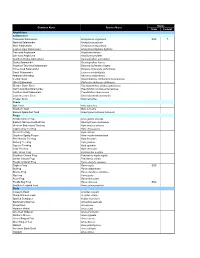
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Gulsby Kennesaw State University
Kennesaw State University DigitalCommons@Kennesaw State University Department of Ecology, Evolution, and Organismal Master of Science in Integrative Biology Theses Biology Summer 7-25-2019 Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Gulsby Kennesaw State University Follow this and additional works at: https://digitalcommons.kennesaw.edu/integrbiol_etd Part of the Integrative Biology Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Gulsby, Miranda, "Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia" (2019). Master of Science in Integrative Biology Theses. 48. https://digitalcommons.kennesaw.edu/integrbiol_etd/48 This Thesis is brought to you for free and open access by the Department of Ecology, Evolution, and Organismal Biology at DigitalCommons@Kennesaw State University. It has been accepted for inclusion in Master of Science in Integrative Biology Theses by an authorized administrator of DigitalCommons@Kennesaw State University. For more information, please contact [email protected]. Ecology of Upland Snake Communities in Managed Montane Longleaf Pine Habitats of Georgia Miranda Louise Gulsby A Thesis Presented in Partial Fulfillment of Requirements of the Master of Science in Integrative Biology for the Department of Evolution, Ecology, and Organismal Biology Kennesaw State University 1000 Chastain Road Kennesaw, Ga 30144 July 2019 Major Advisor: Thomas McElroy, Ph. D. Committee Members: Joel McNeal, Ph. -

Herpetofaunal Communities in Ephemeral Wetlands Embedded Within Longleaf Pine Flatwoods of the Gulf Coastal Plain
20162016 SOUTHEASTERNSoutheastern NaturalistNATURALIST 15(3):431–447Vol. 15, No. 3 K.J. Erwin, H.C. Chandler, J.G. Palis, T.A. Gorman, and C.A. Haas Herpetofaunal Communities in Ephemeral Wetlands Embedded within Longleaf Pine Flatwoods of the Gulf Coastal Plain Kenneth J. Erwin1, Houston C. Chandler1,2,*, John G. Palis3, Thomas A. Gorman1,4, and Carola A. Haas1 Abstract - Ephemeral wetlands surrounded by Pinus palustris (Longleaf Pine) flatwoods support diverse herpetofaunal communities and provide important breeding habitat for many species. We sampled herpetofauna in 3 pine flatwoods wetlands on Eglin Air Force Base, Okaloosa County, FL, over 2 time periods (1 wetland [1] from 1993 to1995 and 2 wetlands [2 and 3] from 2010 to 2015) using drift fences that completely encircled each wetland. We documented 37, 46, and 43 species of amphibians and reptiles at wetlands 1, 2, and 3, respectively. Herpetofaunal communities were remarkably similar across all 3 wetlands (Sorenson Index values > 0.97) despite sampling that occurred 15–20 years apart on wetlands located approximately 10 km apart. Ambystoma bishopi (Reticulated Flat- woods Salamander), Pseudacris ornata (Ornate Chorus Frog), and Eurycea quadridigitata (Dwarf Salamander), all species of conservation concern, were captured at all 3 wetlands, indicating that these wetlands provide habitat for specialist species. Overall, habitat con- servation and management has succeeded in maintaining suitable habitat for herpetofauna in recently surveyed wetlands, despite continued range-wide threats from changes to his- toric fire regimes and climate change. Introduction Ephemeral wetlands often support diverse amphibian and reptile communities (Dodd and Cade 1998, Gibbons et al. 2006, Means et al. -

Microevolution: Species Concept Core Course: ZOOL3014 B.Sc. (Hons’): Vith Semester
Microevolution: Species concept Core course: ZOOL3014 B.Sc. (Hons’): VIth Semester Prof. Pranveer Singh Clines A cline is a geographic gradient in the frequency of a gene, or in the average value of a character Clines can arise for different reasons: • Natural selection favors a slightly different form along the gradient • It can also arise if two forms are adapted to different environments separated in space and migration (gene flow) takes place between them Term coined by Julian Huxley in 1838 Geographic variation normally exists in the form of a continuous cline A sudden change in gene or character frequency is called a stepped cline An important type of stepped cline is a hybrid zone, an area of contact between two different forms of a species at which hybridization takes place Drivers and evolution of clines Two populations with individuals moving between the populations to demonstrate gene flow Development of clines 1. Primary differentiation / Primary contact / Primary intergradation Primary differentiation is demonstrated using the peppered moth as an example, with a change in an environmental variable such as sooty coverage of trees imposing a selective pressure on a previously uniformly coloured moth population This causes the frequency of melanic morphs to increase the more soot there is on vegetation 2. Secondary contact / Secondary intergradation / Secondary introgression Secondary contact between two previously isolated populations Two previously isolated populations establish contact and therefore gene flow, creating an