Group 1 Exhibit 2)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecoregions of New England Forested Land Cover, Nutrient-Poor Frigid and Cryic Soils (Mostly Spodosols), and Numerous High-Gradient Streams and Glacial Lakes
58. Northeastern Highlands The Northeastern Highlands ecoregion covers most of the northern and mountainous parts of New England as well as the Adirondacks in New York. It is a relatively sparsely populated region compared to adjacent regions, and is characterized by hills and mountains, a mostly Ecoregions of New England forested land cover, nutrient-poor frigid and cryic soils (mostly Spodosols), and numerous high-gradient streams and glacial lakes. Forest vegetation is somewhat transitional between the boreal regions to the north in Canada and the broadleaf deciduous forests to the south. Typical forest types include northern hardwoods (maple-beech-birch), northern hardwoods/spruce, and northeastern spruce-fir forests. Recreation, tourism, and forestry are primary land uses. Farm-to-forest conversion began in the 19th century and continues today. In spite of this trend, Ecoregions denote areas of general similarity in ecosystems and in the type, quality, and 5 level III ecoregions and 40 level IV ecoregions in the New England states and many Commission for Environmental Cooperation Working Group, 1997, Ecological regions of North America – toward a common perspective: Montreal, Commission for Environmental Cooperation, 71 p. alluvial valleys, glacial lake basins, and areas of limestone-derived soils are still farmed for dairy products, forage crops, apples, and potatoes. In addition to the timber industry, recreational homes and associated lodging and services sustain the forested regions economically, but quantity of environmental resources; they are designed to serve as a spatial framework for continue into ecologically similar parts of adjacent states or provinces. they also create development pressure that threatens to change the pastoral character of the region. -
Dry River Wilderness
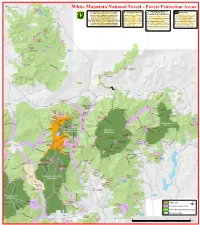
«¬110 SOUTH White Mountain National Forest - Forest Protection Areas POND !5 !B Forest Protection Areas (FPAs) are geographic South !9 Designated Sites !9 The Alpine Zone Wilderness Pond areas where certain activities are restricted to A Rarity in the Northeast Rocky prevent overuse or damage to National Forest Designated sites are campsites or Wilderness Areas are primitive areas Pond resources. Restrictions may include limits on picnic areas within a Forest The alpine zone is a high elevation area in with few signs or other developments. !B camping, use of wood or charcoal fires and Protection area where otherwise which trees are naturally absent or stunted Trails may be rough and difficult to maximum group size. FPAs surround certain features prohibited activities (camping at less that eight feet tall. About 8 square follow. Camping and fires are gererally miles of this habitat exists in the prohibited within 200 feet of any trail W (trails, ponds, parking areas, etc) with either a 200-foot and/or fires) may occur. These e s or ¼ mile buffer. They are marked with signs sites are identified by an official Northeast with most of it over 4000 feet unless at a designated site. No more t M as you enter and exit so keep your eyes peeled. sign, symbol or map. in elevation. Camping is prohibited in the than ten people may occupy a single i la TYPE Name GRID n alpine zone unless there is two or more campsite or hike in the same party. Campgrounds Basin H5 feet of snow. Fires are prohibited at all Big Rock E7 !B Blackberry Crossing G8 ROGERS times. -

Évolution Spatio-Temporelle Du Pergélisol Alpin Marginal Au Mont Jacques-Cartier, Massif Des Chic-Chocs, Gaspésie (Qc)
Université de Montréal Évolution spatio-temporelle du pergélisol alpin marginal au mont Jacques-Cartier, massif des Chic-Chocs, Gaspésie (Qc) par Gautier DAVESNE Département de Géographie Faculté des arts et sciences Mémoire présenté à la Faculté des arts et des sciences en vue de l’obtention du grade de maîtrise en géographie Août 2015 © Gautier Davesne, 2015 ii Université de Montréal Faculté des études supérieures et postdoctorales Ce mémoire intitulé : Évolution spatio-temporelle du pergélisol alpin marginal au mont Jacques-Cartier, massif des Chic- Chocs, Gaspésie (Qc) Présenté par : Gautier Davesne a été évalué par un jury composé des personnes suivantes : François Cavayas, président rapporteur Daniel Fortier, directeur de recherche James Gray, membre du jury iii iv Résumé L’objectif de ce mémoire est d’acquérir une connaissance détaillée sur l’évolution spatiale de la température de surface du sol (GST) au mont Jacques-Cartier et sur la réponse thermique de son îlot de pergélisol alpin aux changements climatiques passés et futurs. L’étude est basée sur un ensemble de mesures de température (GST, sous-sol) et de neige, ainsi que des modèles spatiaux de distribution potentielle de la GST et des simulations numériques du régime thermique du sol. Les résultats montrent que la distribution de la GST sur le plateau est principalement corrélée avec la répartition du couvert nival. Au-dessus de la limite de la végétation, le plateau est caractérisé par un couvert de neige peu épais et discontinu en hiver en raison de la topographie du site et l’action des forts vents. La GST est alors couplée avec les températures de l’air amenant des conditions froides en surface. -

Taiga Plains
ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES Taiga Plains Ecosystem Classification Group Department of Environment and Natural Resources Government of the Northwest Territories Revised 2009 ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES TAIGA PLAINS This report may be cited as: Ecosystem Classification Group. 2007 (rev. 2009). Ecological Regions of the Northwest Territories – Taiga Plains. Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, NT, Canada. viii + 173 pp. + folded insert map. ISBN 0-7708-0161-7 Web Site: http://www.enr.gov.nt.ca/index.html For more information contact: Department of Environment and Natural Resources P.O. Box 1320 Yellowknife, NT X1A 2L9 Phone: (867) 920-8064 Fax: (867) 873-0293 About the cover: The small photographs in the inset boxes are enlarged with captions on pages 22 (Taiga Plains High Subarctic (HS) Ecoregion), 52 (Taiga Plains Low Subarctic (LS) Ecoregion), 82 (Taiga Plains High Boreal (HB) Ecoregion), and 96 (Taiga Plains Mid-Boreal (MB) Ecoregion). Aerial photographs: Dave Downing (Timberline Natural Resource Group). Ground photographs and photograph of cloudberry: Bob Decker (Government of the Northwest Territories). Other plant photographs: Christian Bucher. Members of the Ecosystem Classification Group Dave Downing Ecologist, Timberline Natural Resource Group, Edmonton, Alberta. Bob Decker Forest Ecologist, Forest Management Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Hay River, Northwest Territories. Bas Oosenbrug Habitat Conservation Biologist, Wildlife Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, Northwest Territories. Charles Tarnocai Research Scientist, Agriculture and Agri-Food Canada, Ottawa, Ontario. Tom Chowns Environmental Consultant, Powassan, Ontario. Chris Hampel Geographic Information System Specialist/Resource Analyst, Timberline Natural Resource Group, Edmonton, Alberta. -

Schedule of Proposed Action (SOPA)
Schedule of Proposed Action (SOPA) 10/01/2019 to 12/31/2019 White Mountain National Forest This report contains the best available information at the time of publication. Questions may be directed to the Project Contact. Expected Project Name Project Purpose Planning Status Decision Implementation Project Contact White Mountain National Forest Androscoggin Ranger District (excluding Projects occurring in more than one District) R9 - Eastern Region Evans Brook Vegetation - Wildlife, Fish, Rare plants In Progress: Expected:01/2020 06/2020 Patricia Nasta Management Project - Forest products Comment Period Public Notice 207-824-2813 EA - Vegetation management 05/15/2019 [email protected] *UPDATED* (other than forest products) Description: Proposed timber harvest using even-aged and uneven-aged management methods to improve forest health, improve wildlife habitat diversity, and provide for a sustainable yield of forest products. Connected road work will be proposed as well. Web Link: http://www.fs.usda.gov/project/?project=52040 Location: UNIT - Androscoggin Ranger District. STATE - Maine. COUNTY - Oxford. LEGAL - Not Applicable. The proposed units are located between Hastings Campground and Evans Notch along Route 113, and west of Route 113 from Bull Brook north. Peabody West Conceptual - Recreation management Developing Proposal Expected:12/2021 01/2022 Johnida Dockens Proposal Development - Wildlife, Fish, Rare plants Est. Scoping Start 11/2019 207-323-5683 EA - Forest products johnida.dockens@usda. - Vegetation management gov (other than forest products) - Road management Description: The Androscoggin Ranger District of the White Mountain National Forest is in the early stages of proposal development for management activities within the conceptual Peabody West area, Coos County, NH. -

An Evaluation of the Wetland and Upland Habitats And
AN EVALUATION OF THE WETLAND AND UPLAND HABITATS AND ASSOCIATED WILDLIFE RESOURCES IN SOUTHERN CANAAN VALLEY CANAAN VALLEY TASK FORCE SUBMl'l*IED BY: EDWIN D. MICHAEL, PH.D. PROFESSOR OF WILDLIFEMANAGEI\fENT DIVISION OF FORESTRY WEST VIRGINIA UNIVERSITY MORGANTOWN, WV 26506 December 1993 TABLB OP' CONTENTS Page EXECUTIVE SUMMARY 1 INTRODUCTION 6 OBJECTIVES 6 PROCEDURES 6 THE STUDY AREA Canaan Valley .... ..... 7 Southern Canaan Valley .... 8 Development and Land Use 8 Existing Environment Hydrology ........ 9 Plant Communities .... 11 1. Northern hardwoods . 11 2. Conifers ... 11 3. Aspen groves . 11 4. Alder thickets 12 5. Ecotone 12 6. Shrub savannah 12 7. Spiraea 13 8. Krummholz 13 9. Bogs ..... 13 10. Beaver ponds 13 11. Agriculture . l4 Vegetation of Southern Canaan Valley Wetlands 14 Rare and Endangered Plant Species 16 Vertebrate Animals 16 1. Fishes .. 16 2. Amphibians 18 3. Reptiles 19 4. Birds 20 5. Mammals 24 Rare and Endangered Animal Species 25 Game Animals 27 Cultural Values 28 Aesthetic Values 31 1. Landform contrast 31 2. Land-use contrast 31 3. Wetland-type diversity 32 4. Internal wetland contrast 32 5. Wetland size ... 32 6. Landform diversity .... 32 DISCUSSION Streams 32 Springs and Spring Seeps 34 Lakes . 35 Wetland Habitats 35 ii Wildlife 36 Management Potential 38 Off-road Vehicle Use 42 Fragmentation . 42 Cultural Values 44 Educational Values SIGNIFICANCE OF THE AREA OF CONCERN FOR FULFILLMENT OF THE CANAAN VALLEY NATIONAL WILDLIFE REFUGE 1979 EIS OBJECTIVES 46 CONCLUSIONS .. 47 LITERATURE CITED 52 TABLES 54 FIGURES 88 iii LIST OF TABLES 1. Property ownerships of Canaan Valley ... ..... 8 2. -

Maine Alumnus, Volume 59, Number 1, Winter 1978
The University of Maine DigitalCommons@UMaine University of Maine Alumni Magazines University of Maine Publications Winter 1978 Maine Alumnus, Volume 59, Number 1, Winter 1978 General Alumni Association, University of Maine Follow this and additional works at: https://digitalcommons.library.umaine.edu/alumni_magazines Part of the Higher Education Commons, and the History Commons Recommended Citation General Alumni Association, University of Maine, "Maine Alumnus, Volume 59, Number 1, Winter 1978" (1978). University of Maine Alumni Magazines. 301. https://digitalcommons.library.umaine.edu/alumni_magazines/301 This publication is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in University of Maine Alumni Magazines by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. * be back in Maine... To once again savor its good life and now share that exhilarating experience with my family. Io both entertain and serve all who love this great State by continuing publication of the Magazine of Maine along the lines developed over the past twenty-three years by its founder — now editor emeritus — Duane Doolittle. Io join Down East Editor Dave Thomas in maintaining established standards of recalling Maine's fascinating past, reporting her vital pre sent, and revealing the potential of her future. And to improve upon those respected standards where possible. [t's an exciting challenge and one which, we believe, has already been excitingly engaged — both in picture and in word — to make Down East now — more than ever before — The Magazine of Maine. And now Down East is a Maine excitement you can enjoy eleven times a year instead of ten. -

2018 White Mountains of Maine
2018 White Mountains of Maine Summit Handbook 2018 White Mountains of Maine Summit Welcome to the 2018 Family Nature Summit! We are thrilled that you have chosen to join us this summer at the Sunday River Resort in the White Mountains of Maine! Whether this is your first time or your fifteenth, we know you appreciate the unparalleled value your family receives from attending a Family Nature Summit. One of the aspects that is unique about the Family Nature Summits program is that children have their own program with other children their own age during the day while the adults are free to choose their own classes and activities. Our youth programs are run by experienced and talented environmental educators who are very adept at providing a fun and engaging program for children. Our adult classes and activities are also taught by experts in their fields and are equally engaging and fun. In the afternoon, there are offerings for the whole family to do together as well as entertaining evening programs. Family Nature Summits is fortunate to have such a dedicated group of volunteers who have spent countless hours to ensure this amazing experience continues year after year. This handbook is designed to help orient you to the 2018 Family Nature Summit program. We look forward to seeing you in Maine! Page 2 2018 White Mountains of Maine Summit Table of Contents Welcome to the 2018 Family Nature Summit! 2 Summit Information 7 Summit Location 7 Arrival and Departure 7 Room Check-in 7 Summit Check-in 7 Group Picture 8 Teacher Continuing Education -

Characteristics of the Ecoregions of New England 5 8
Summary Table: Characteristics of the Ecoregions of New England 5 8 . NORTHEASTERN HIGHLANDS 5 9 . NORTHEASTERN COASTAL ZONE Level IV Ecoregions Physiography Geology Soils Climate Natural Vegetation Land Cover and Land Use Level IV Ecoregions Physiography Geology Soils Climate Natural Vegetation Land Cover and Land Use Area Elevation / Surficial and Bedrock Order (Great Group) Common Soil Series Temperature / Precipitation Frost Free Mean Temperature Area Elevation / Surficial and Bedrock Order (Great Group) Common Soil Series Temperature / Precipitation Frost Free Mean Temperature (square Local Relief Moisture Mean annual Mean annual January min/max; (square Local Relief Moisture Mean annual Mean annual January min/max; miles) (feet) Regimes (inches) (days) July min/max (oF) miles) (feet) Regimes (inches) (days) July min/max (oF) 58a. Taconic 584 Low mountains and high hills, gently 600-3816 / Quaternary loamy till and sandy loamy till, valley Inceptisols (Dystrudepts) Taconic, Macomber, Frigid / 38-64 100-140 10/28; Southern-influenced forests with oaks and hickories on lower and drier slopes, including Deciduous forest, some minor pasture 59a. Connecticut 1459 Level to rolling plains with some high 10-1106 (Mt. Holocene alluvium. Quaternary deposits mostly Entisols (Udifluvents, Hadley, Hinckley, Limerick, Mesic / 38-52 135-180 16/35; Mostly central and transition hardwoods. Mixed oak and oak-conifer forests including Urban, suburban, and rural residential, rounded to steep slopes, narrow valleys. 800-2000 bottom deposits of alluvium. -

Speckled Mountain Heritage Hikes Bickford Brook and Blueberry Ridge Trails – 8.2-Mile Loop, Strenuous
Natural Speckled Mountain Heritage Hikes Bickford Brook and Blueberry Ridge Trails – 8.2-mile loop, strenuous n the flanks and summit of Speckled Mountain, human and natural history mingle. An old farm Oroad meets a fire tower access path to lead you through a menagerie of plants, animals, and natural communities. On the way down Blueberry Ridge, 0with0.2 a sea 0.4 of blueberry 0.8 bushes 1.2 at 1.6 your feet and spectacular views around every bend, the rewards of the summit seem endless. Miles Getting There Click numbers to jump to descriptions. From US Route 2 in Gilead, travel south on Maine State Route 113 for 10 miles to the parking area at Brickett Place on the east side of the road. From US Route 302 in Fryeburg, travel north on Maine State Route 113 for 19 miles to Brickett Place. 00.2 0.4 0.8 1.2 1.6 Miles A House of Many Names -71.003608, 44.267292 Begin your hike at Brickett Place Farm. In the 1830s, a century before present-day Route 113 sliced through Evans Notch, John Brickett and Catherine (Whitaker) Brickett built this farmhouse from home- made bricks. Here, they raised nine children alongside sheep, pigs, cattle, and chick- ens. Since its acquisition by the White Mountain National Forest in 1918, Brickett Place Farmhouse has served as Civilian Conservation Corps headquarters (1930s), Cold River Ranger Station (1940s), an Appalachian Mountain Club Hut (1950s), and a Boy Scouts of America camp (1960-1993). Today, Brickett Place Farmhouse has gone through a thorough restoration and re- mains the oldest structure in the eastern region of the Forest Service. -

Amc Cold River Camp
AMC COLD RIVER CAMP NORTH CHATHAM, NEW HAMPSHIRE Winter 2018 ◊ Number 37 www.amccoldrivercamp.org 44 ˚ 14’ 10.1” N 71 ˚ 0’ 42.8” W CHAIRMAN’S WELCOME Dover, New Hampshire, January 2018 Dear Cold River Camp community, ctober 27, 2017. Needle ice. Those exquisite, fragile crystalline columns of ice that grow out of the ground when conditions are right – mois- Oture-saturated unfrozen dirt and the air temperature below freezing. Beth and I were headed up the A-Z and Mount Tom Spur trails to the Mount Tom summit. Not much of a view to be had up there, but what a special hike! A couples miles of trail whose sides were strewn with oodles of patches and swaths of needle ice. A fairyland of sorts. You just never know what treasure the next hike will reveal. July 1, 2018. That day will mark the one hundred years duration of Cold River Camp’s operation serving guests as an Appalachian Mountain Club facility. Imagine that! All the guest footsteps walking our same trails. All the conver- sations at meals. The songs, poems and skits evoked and shared on Talent Nights. And volunteer-managed all that time. It is marvelous that so many of our CRC community carry on that tradition of committed investment. But we’ll hold our enthusiasm in check and do our celebrating in our 100th anniversary year, which will be 2019. A celebration committee has been at hard at work for about two years. You can read more about that on page 18 of this LDD. -

Building a Sustainable Natural Resource-Based Economy
Blaine House Conference on Maine’s Natural Resource-based Industry: Charting a New Course November 17, 2003 onference Report With recommendations to Governor John E. Baldacci Submitted by the Conference Planning Committee Richard Barringer and Richard Davies, Co-chairs February 2004 . Acknowledgements The following organizations made the conference possible with their generous contributions: Conference Sponsors The Betterment Fund Finance Authority of Maine LL Bean, Inc. Maine Community Foundation US Fish and Wildlife Service US Forest Service This report was compiled and edited by: Maine State Planning Office 38 State House Station Augusta, ME 04333 (207) 287-3261 www.maine/gov/spo February 2004 Printed under Appropriation #014 07B 3340 2 . Conference Planning Committee Richard Barringer, Co-chair, Richard Davies, Co-chair Professor, Muskie School of Public Service, Senior Policy Advisor, Governor Office USM Dann Lewis Spencer Apollonio Director, Office of Tourism, DECD Former Commissioner, Dept of Marine Resources Roland D. Martin Commissioner, Dept of Inland Fisheries and Edward Bradley Wildlife Maritime Attorney Patrick McGowan Elizabeth Butler Commissioner, Dept of Conservation Pierce Atwood Don Perkins Jack Cashman President, Gulf of Maine Research Institute Commissioner, Dept of Economic and Community Development Stewart Smith Professor, Sustainable Agriculture Policy, Charlie Colgan UMO Professor, Muskie School of Public Service, USM Robert Spear Commissioner, Dept of Agriculture Martha Freeman Director, State Planning Office