Symbols, Formulas and Molar Masses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of the Molar Mass of an Unknown Solid by Freezing Point Depression
Determination of the Molar Mass of an Unknown Solid by Freezing Point Depression GOAL AND OVERVIEW In the first part of the lab, a series of solutions will be made in order to determine the freezing point depression constant, Kf, for cyclohexane. The freezing points of these solutions, which will contain known amounts of p-dichlorobenzene dissolved in cyclohexane, will be measured. In the second part of the lab, the freezing point of a cyclohexane solution will be prepared that contains a known mass of an unknown organic solid. The measured freezing point change will be used to calculate the molar mass of the unknown solid. Objectives and Science Skills • Understand and explain colligative properties of solutions, including freezing point depression. • Measure the freezing point of a pure solvent and of a solution in that solvent containing an unknown solute. • Calculate the molar mass of the unknown solute and determine its probable identity from a set of choices. • Quantify the expected freezing point change in a solution of known molality. • Quantitatively and qualitatively compare experimental results with theoretical values. • Identify and discuss factors or effects that may contribute to the uncertainties in values determined from experimental data. SUGGESTED REVIEW AND EXTERNAL READING • Reference information on thermodynamics; data analysis; relevant textbook information BACKGROUND If a liquid is at a higher temperature than its surroundings, the liquid's temperature will fall as it gives up heat to the surroundings. When the liquid's temperature reaches its freezing point, the liquid's temperature will not fall as it continues to give up heat to the surroundings. -

Δtb = M × Kb, Δtf = M × Kf
8.1HW Colligative Properties.doc Colligative Properties of Solvents Use the Equations given in your notes to solve the Colligative Property Questions. ΔTb = m × Kb, ΔTf = m × Kf Freezing Boiling K K Solvent Formula Point f b Point (°C) (°C/m) (°C/m) (°C) Water H2O 0.000 100.000 1.858 0.521 Acetic acid HC2H3O2 16.60 118.5 3.59 3.08 Benzene C6H6 5.455 80.2 5.065 2.61 Camphor C10H16O 179.5 ... 40 ... Carbon disulfide CS2 ... 46.3 ... 2.40 Cyclohexane C6H12 6.55 80.74 20.0 2.79 Ethanol C2H5OH ... 78.3 ... 1.07 1. Which solvent’s freezing point is depressed the most by the addition of a solute? This is determined by the Freezing Point Depression constant, Kf. The substance with the highest value for Kf will be affected the most. This would be Camphor with a constant of 40. 2. Which solvent’s freezing point is depressed the least by the addition of a solute? By the same logic as above, the substance with the lowest value for Kf will be affected the least. This is water. Certainly the case could be made that Carbon disulfide and Ethanol are affected the least as they do not have a constant. 3. Which solvent’s boiling point is elevated the least by the addition of a solute? Water 4. Which solvent’s boiling point is elevated the most by the addition of a solute? Acetic Acid 5. How does Kf relate to Kb? Kf > Kb (fill in the blank) The freezing point constant is always greater. -

Find the Molar Mass of Sodium Carbonate, Na 2CO3. Na 2 X
Moles and Molar Mass The mole is the "counting unit" used by chemists to indicate the number of atoms, ions, molecules, or formula units present in a particular chemical sample. The mole is similar to other counting units that you've used before....pair (2), dozen (12), and gross (144). One mole of a compound contains Avogadro's number (6.022 x 1023) of molecules (molecular compound) or formula units (ionic compound). The molar mass of a compound tells you the mass of 1 mole of that substance. In other words, it tells you the number of grams per mole of a compound. The units for molar mass are, therefore, grams/mole. To find the molar mass of a compound: 1. Use the chemical formula to determine the number of each type of atom present in the compound. 2. Multiply the atomic weight (from the periodic table) of each element by the number of atoms of that element present in the compound. 3. Add it all together and put units of grams/mole after the number. Example: Find the molar mass of sodium carbonate, Na2CO3. Na 2 x 23.0 = 46.0 C 1 x 12.0 = 12.0 O 3 x 16.0 = 48.0 molar = 106.0 g/mole mass For many (but not all) problems, you can simply round the atomic weights and the molar mass to the nearest 0.1 g/mole. HOWEVER, make sure that you use at least as many significant figures in your molar mass as the measurement with the fewest significant figures. -

Atomic Weights and Isotopic Abundances*
Pure&App/. Chem., Vol. 64, No. 10, pp. 1535-1543, 1992. Printed in Great Britain. @ 1992 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY INORGANIC CHEMISTRY DIVISION COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCES* 'ATOMIC WEIGHT' -THE NAME, ITS HISTORY, DEFINITION, AND UNITS Prepared for publication by P. DE BIEVRE' and H. S. PEISER2 'Central Bureau for Nuclear Measurements (CBNM), Commission of the European Communities-JRC, B-2440 Geel, Belgium 2638 Blossom Drive, Rockville, MD 20850, USA *Membership of the Commission for the period 1989-1991 was as follows: J. R. De Laeter (Australia, Chairman); K. G. Heumann (FRG, Secretary); R. C. Barber (Canada, Associate); J. CCsario (France, Titular); T. B. Coplen (USA, Titular); H. J. Dietze (FRG, Associate); J. W. Gramlich (USA, Associate); H. S. Hertz (USA, Associate); H. R. Krouse (Canada, Titular); A. Lamberty (Belgium, Associate); T. J. Murphy (USA, Associate); K. J. R. Rosman (Australia, Titular); M. P. Seyfried (FRG, Associate); M. Shima (Japan, Titular); K. Wade (UK, Associate); P. De Bi&vre(Belgium, National Representative); N. N. Greenwood (UK, National Representative); H. S. Peiser (USA, National Representative); N. K. Rao (India, National Representative). Republication of this report is permitted without the need for formal IUPAC permission on condition that an acknowledgement, with full reference together with IUPAC copyright symbol (01992 IUPAC), is printed. Publication of a translation into another language is subject to the additional condition of prior approval from the relevant IUPAC National Adhering Organization. ’Atomic weight‘: The name, its history, definition, and units Abstract-The widely used term “atomic weight” and its acceptance within the international system for measurements has been the subject of debate. -

2.1 Relative Atomic and Molecular Masses, the Avogadro Constant, and the Mole Calculations AQA Chemistry
2.1 Relative atomic and molecular AQA Chemistry masses, the Avogadro constant, and the mole Calculations The Avogadro constant Specification reference 3.1.2.2 MS 0.1 Recognise and use expressions in decimal and ordinary form MS 0.4 Use calculators to find and use power, exponential, and logarithmic functions MS 1.1Use an appropriate number of significant figures. Learning objectives After completing the worksheet you should be able to: carry out calculations using the Avogadro constant carry out calculations using numbers in standard and ordinary form substitute numerical values into algebraic equations, and change the subject of equations report calculations to an appropriate number of significant figures. Introduction One mole of any substance contains the same number of particles as the number of atoms in 12 g of carbon-12. This number of particles is a huge number and is called the Avogadro constant. It has a value of 6.02 1023 and is given the units of mol−1 (per mole). The relative atomic mass of an element in grams contains 6.02 1023 atoms or one mole of that element. The relative formula mass of a compound in grams contains 6.02 1023 particles (molecules or ions) or one mole of that compound. Therefore we use the term molar mass to describe the relative atomic or formula mass of a substance in grams and give it the unit g mol−1. To calculate the number of particles in a certain mass of a substance we can use the following equation. mass m (g) Number of particles = ´ 6.02 ´ 1023(mol-1) molar mass M (g mol-1) Worked example Question 1 How many molecules are there in 42.5 g of ammonia, NH3? Answer © Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original 1 2.1 Relative atomic and molecular AQA Chemistry masses, the Avogadro constant, and the mole Calculations Step 1 Calculate the molar mass of ammonia using the relative atomic masses of nitrogen and hydrogen from the periodic table. -

Nuclear Criticality Safety Engineer Training Module 1 1
Nuclear Criticality Safety Engineer Training Module 1 1 Introductory Nuclear Criticality Physics LESSON OBJECTIVES 1) to introduce some background concepts to engineers and scientists who do not have an educational background in nuclear engineering, including the basic ideas of moles, atom densities, cross sections and nuclear energy release; 2) to discuss the concepts and mechanics of nuclear fission and the definitions of fissile and fissionable nuclides. NUCLEAR CRITICALITY SAFETY The American National Standard for Nuclear Criticality Safety in Operations with Fissionable Materials Outside Reactors, ANSI/ANS-8.1 includes the following definition: Nuclear Criticality Safety: Protection against the consequences of an inadvertent nuclear chain reaction, preferably by prevention of the reaction. Note the words: nuclear - related to the atomic nucleus; criticality - can it be controlled, will it run by itself; safety - protection of life and property. DEFINITIONS AND NUMBERS What is energy? Energy is the ability to do work. What is nuclear energy? Energy produced by a nuclear reaction. What is work? Work is force times distance. 1 Developed for the U. S. Department of Energy Nuclear Criticality Safety Program by T. G. Williamson, Ph.D., Westinghouse Safety Management Solutions, Inc., in conjunction with the DOE Criticality Safety Support Group. NCSET Module 1 Introductory Nuclear Criticality Physics 1 of 18 Push a car (force) along a road (distance) and the car has energy of motion, or kinetic energy. Climb (force) a flight of steps (distance) and you have energy of position relative to the first step, or potential energy. Jump down the stairs or out of a window and the potential energy is changed to kinetic energy as you fall. -

Answer Key Chapter 6: Standard Review Worksheet 1
Answer Key Chapter 6: Standard Review Worksheet 1. 1 amu = 1.66 _ 10–24 g. For example, the average atomic mass of sodium is 22.99 amu, which represents the average mass of all the sodium atoms in the world (including all the various isotopes and their relative abundances). So that we will be able to use the mass of a sample of sodium to count the number of atoms of sodium present in the sample, we consider that every sodium atom in a sample has exactly the same mass (the average atomic mass). The average atomic mass of an element is typically not a whole number of amu’s because of the presence of the different isotopes of the element, each with its own relative abundance. Since the relative abundance of an element can be any number, when the weighted average atomic mass of the element is calculated, the average is unlikely to be a whole number. 2. On a microscopic basis, one mole of a substance represents Avogadro’s number (6.022 _ 1023) of individual units (atoms or molecules) of the substance. On a macroscopic basis, one mole of a substance represents the amount of substance present when the molar mass of the substance in grams is taken (for example, 12.01 g of carbon will be one mole of carbon). 3. The molar mass of a compound is the mass in grams of one mole of the compound (6.022 _ 1023 molecules of the compound) and is calculated by summing the average atomic masses of all the atoms present in a molecule of the compound. -

5.1: Atomic Mass Unit 5.1: Atomic Mass
Topic 5: Stoichiometry - Chemical Arithmetic Masses of some atoms: 1 -24 16 -23 1H = 1.6736×10 g 8 O = 2.6788 ×10 g 238 -22 92U = 3.9851×10 g Introducing…….the Atomic Mass Unit (amu) 1 amu = 1.66054 x 10-24 g 1 5.1: Atomic Mass Unit Atomic Mass is defined relative to Carbon -12 isotope 12 12 amu is the mass of the 6 C isotope of carbon Carbon -12 atom = 12.000 amu Hydrogen -1 atom = 1.008 amu Oxygen -16 atom = 15.995 amu Chlorine -35 atom = 34.969 amu 2 5.1: Atomic Mass - Natural Abundance We deal with the naturally occurring mix of isotopes, rather than pure isotopes Carbon has three natural isotopes Isotope Mass (amu) Abundance (%) 12C 12.000 98.892 13C 13.00335 1.108 14C 14.00317 1 x 10-4 Any shovelful of Carbon from living material will have a Naturally Occurring Abundance of 98.892% 12C, 1.108% 13C and 0.0001% 14C 3 1 5.1: Atomic Mass - Relative Abundance How do we take into account the naturally occurring Abundances? Take the average mass of the various isotopes weighted according to their Relative Abundances Relative Isotope Mass (amu) Abundance (%) Abundance 12C 12.000 98.892 0.98892 13C 13.00335 1.108 0.0108 -6 14C 14.00317 1 x 10-4 1 x 10 N.B. The % Abundance adds up to 100 The Relative Abundance adds up to 1 4 5.1: Average Atomic Mass Relative Isotope Mass (amu) Abundance (%) Abundance 12C 12.000 98.892 0.98892 13C 13.00335 1.108 0.0108 -6 14C 14.00317 1 x 10-4 1 x 10 The Average Atomic Mass is given by: (0.98892 x 12.000 amu) + (0.01108 x 13.00335 amu) + (1 x 10-6 x 14.00317 amu) = 12.011 amu 5 5.1: Atomic and Molecular Mass You can calculate the mass of any compound from the sum of the atomic masses from the periodic table. -

Make an Atom Vocabulary Grade Levels
MAKE AN ATOM Fundamental to physical science is a basic understanding of the atom. Atoms are comprised of protons, neutrons, and electrons. Protons and neutrons are at the center of the atom while electrons live in lobe-shaped clouds outside the nucleus. The number of electrons usually matches the number of protons, yielding a net neutral charge for the atom. Sometimes an atom has less neutrons or more neutrons than protons. This is called an isotope. If an atom has different numbers of electrons than protons, then it is an ion. If an atom has different numbers of protons, it is a different element all together. Scientists at Idaho National Laboratory study, create, and use radioactive isotopes like Uranium 234. The 234 means this isotope has an atomic mass of 234 Atomic Mass Units (AMU). GRADE LEVELS: 3-8 VOCABULARY Atom – The basic unit of a chemical element. Proton – A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron, but of opposite sign. Neutron – A subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. Electron – A stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids. Orbital – Each of the actual or potential patterns of electron density that may be formed on an atom or molecule by one or more electrons. Ion – An atom or molecule with a net electric charge due to the loss or gain of one or more electrons. -

Experiment 9
Experiment Molar Mass by Vapor Density 7 Vapor is the term for a gas produced from the vaporization of a liquid. If it is assumed that the resulting vapor is an ideal gas, then we can use the ideal gas law: By substituting moles for mass and molar mass the following equation is derived after some simple rearrangements: The molar mass of the compound is represented by Mm and d is the vapor density in g per liter. Another way to calculate the molar mass of the sample is to determine the moles of the gas produced. The number of moles can be determined from the measured volume, pressure, ad temperature of the gas. From the mass of the liquid sample used and the moles calculated from experimental measurements, the molar mass of the sample can be determined. Many organic compounds are liquids that vaporized below the boiling point of water. A boiling water bath will be used as a constant temperature (100 C or 373 K) heat source to convert your unknown liquid to a gas. The pressure of the gas will be the barometric pressure of the room and the volume is the volume of the gas inside the container (flask). In this experiment, you will be given an unknown liquid and you will determine the molar mass of the liquid. Equipment and Reagents Analytical balance unknown liquid high capacity balance 200 mL flask tap water thermometer Aluminum foil stand w/ iron ring barometer Pin wire gauze 600 mL beaker buret clamp 1 Procedure 1. On an analytical balance weigh a 200 mL Erlenmeyer flask with a piece of aluminum foil large enough to cover the mouth of the flask. -
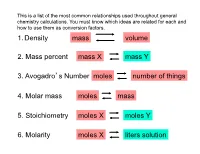
1. Density Mass Volume 3. Avogadro's Number Moles
This is a list of the most common relationships used throughout general chemistry calculations. You must know which ideas are related for each and how to use them as conversion factors. 1. Density mass volume 2. Mass percent mass X mass Y 3. Avogadro’s Number moles number of things 4. Molar mass moles mass 5. Stoichiometry moles X moles Y 6. Molarity moles X liters solution 1. Density mass volume • convert from mass to volume 6.54 g 1 cm3 = 8.32 cm3 = 8.32 mL 0.7857 g • convert from volume to mass 28.56 cm3 0.7857 g = 22.44 g 1 cm3 • calculate density directly density = 371 g = 19.2 g/mL 19.3 cm3 This is a list of the most common relationships used throughout general chemistry calculations. You must know which ideas are related for each and how to use them as conversion factors. 1. Density mass volume 2. Mass percent mass X mass Y 3. Avogadro’s Number moles number of things 4. Molar mass moles mass 5. Stoichiometry moles X moles Y 6. Molarity moles X liters solution 2. Mass percent mass X mass Y 55.5 g CuF2 37.42 g F = 20.8 g F 100 g CuF2 This is a list of the most common relationships used throughout general chemistry calculations. You must know which ideas are related for each and how to use them as conversion factors. 1. Density mass volume 2. Mass percent mass X mass Y 3. Avogadro’s Number moles number of things 4. Molar mass moles mass 5. -

Molar Mass 1 Molar Mass
Molar mass 1 Molar mass In chemistry, the molar mass is a physical property. It is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. The base SI unit for molar mass is kg/mol. However, for historical reasons, molar masses are almost always expressed in g/mol. As an example, the molar mass of water is approximately: M(H O) ≈ 18 g⋅mol−1 2 Molar masses of elements The molar mass of atoms of an element is given by the atomic mass of the element multiplied by the molar mass constant, M −3 u = 1×10 kg/mol = 1 g/mol: M(H) = 1.007 97(7) × 1 g/mol = 1.007 97(7) g/mol M(S) = 32.065(5) × 1 g/mol = 32.065(5) g/mol M(Cl) = 35.453(2) × 1 g/mol = 35.453(2) g/mol M(Fe) = 55.845(2) × 1 g/mol = 55.845(2) g/mol. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole). Some elements are usually encountered as molecules, e.g. hydrogen (H 2), sulfur (S 8), chlorine (Cl 2). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule: M(H 2) = 2 × 1.007 97(7) × 1 g/mol = 2.015 88(14) g/mol M(S 8) = 8 × 32.065(5) × 1 g/mol = 256.52(4) g/mol M(Cl 2) = 2 × 35.453(2) × 1 g/mol = 70.906(4) g/mol.