2.1 Relative Atomic and Molecular Masses, the Avogadro Constant, and the Mole Calculations AQA Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of the Molar Mass of an Unknown Solid by Freezing Point Depression
Determination of the Molar Mass of an Unknown Solid by Freezing Point Depression GOAL AND OVERVIEW In the first part of the lab, a series of solutions will be made in order to determine the freezing point depression constant, Kf, for cyclohexane. The freezing points of these solutions, which will contain known amounts of p-dichlorobenzene dissolved in cyclohexane, will be measured. In the second part of the lab, the freezing point of a cyclohexane solution will be prepared that contains a known mass of an unknown organic solid. The measured freezing point change will be used to calculate the molar mass of the unknown solid. Objectives and Science Skills • Understand and explain colligative properties of solutions, including freezing point depression. • Measure the freezing point of a pure solvent and of a solution in that solvent containing an unknown solute. • Calculate the molar mass of the unknown solute and determine its probable identity from a set of choices. • Quantify the expected freezing point change in a solution of known molality. • Quantitatively and qualitatively compare experimental results with theoretical values. • Identify and discuss factors or effects that may contribute to the uncertainties in values determined from experimental data. SUGGESTED REVIEW AND EXTERNAL READING • Reference information on thermodynamics; data analysis; relevant textbook information BACKGROUND If a liquid is at a higher temperature than its surroundings, the liquid's temperature will fall as it gives up heat to the surroundings. When the liquid's temperature reaches its freezing point, the liquid's temperature will not fall as it continues to give up heat to the surroundings. -

Avogadro Number, Boltzmann Constant, Planck Constant, Information Theory, Comparative and Relative Uncertainties
American Journal of Computational and Applied Mathematics 2018, 8(5): 93-102 DOI: 10.5923/j.ajcam.20180805.02 h, k, NA: Evaluating the Relative Uncertainty of Measurement Boris Menin Mechanical & Refrigeration Consultation Expert, Beer-Sheba, Israel Abstract For verification of the measurement accuracy of fundamental physical constants, the CODATA unique technique is used. This procedure is a complex process based on a careful discussion of the input data, and the justification and construction of tables of values sufficient for the direct use of the relative uncertainty are conducted using modern advanced statistical methods and powerful computers. However, at every stage of data processing, researchers must rely on common sense, that is, an expert conclusion. In this article, the author proposes a theoretical and informational grounded justification for calculating the relative uncertainty by using the comparative uncertainty. A detailed description of the data and the processing procedures do not require considerable time. Examples of measurements results of three fundamental constants are analysed, applying information-oriented approach. Comparison of the achieved relative and comparative uncertainties is introduced and discussed. Keywords Avogadro number, Boltzmann constant, Planck constant, Information theory, Comparative and relative uncertainties theories of physics can be proved by carefully studying the 1. Introduction numerical values of these constants, determined from different experiments in different fields of physics. It is expected that in 2018 that the International System of One needs to note the main feature of the CODATA Units (SI) will be redefined on the basis of certain values of technique in determining the relative uncertainty of one some fundamental constants [1]. -

X-Ray Crystal Density Method to Determine the Avogadro and Planck Constants
X-ray Crystal Density Method to Determine the Avogadro and Planck Constants Horst Bettin Physikalisch-Technische Bundesanstalt Braunschweig, Germany Proposed New Definition of the Kilogram The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 040 x 10 -34 when expressed in the unit J s, which is equal to kg m2 s-1, where the metre and the second c ∆ν are defined in terms of and Cs . *) X represents one or more digits to be added at the time the new definition is finally adopted. α 2 - = M (e )c NAh 2 R∞ N h −10 A = 3.990 312 7110(18) × 10 Js/mol, Amedeo Avogadro Max Planck with relative uncertainty of 0.45 × 10 -9 (1776-1856) (1858-1947) Page 2 of 19 Avogadro Constant Definition of Avogadro constant NA • Number of molecules per mol • 6.022... x 10 23 mol -1 Amedeo Avogadro Current definition of mol (1776-1856) • Number “entities” like 12 C atoms in 12 g • i. e. 6.022... x 10 23 12 C atoms have a mass of 12 g 12 12 g/mol = NA m( C ) Faraday constant F = NA e (e: elementary charge) Molar gas constant R = NA k (k: Boltzmann constant) Page 3 of 19 Counting Atoms: XRCD Method 3 Use of a silicon crystal! 1. Volume a0 of the unit cell 3 2. Volume of an atom: a0 /8 3. Volume V of a sphere 4. Number N of the atoms 8 V8 VM N = = ⋅ mol A N 3 3 a0 a0msphere Page 4 of 19 Lattice parameter measurement (INRIM) d220 (2011)=192014712.67(67) am d220 (2014)=192014711.98(34) am -9 ur(2014) = 1.8 x 10 Page 5 of 19 Lattice parameter set-up at PTB S M1 M M2 AL AA OH Page 6 of 19 Sphere Interferometer of PTB mK-temperature stabilisation Camera 1 Camera 2 Fizeau- Fizeau- Collimator Objective 1 Objective 2 Diode laser Page 7 of 19 Diameter results (2014) Relative Mean apparent Sphere Lab. -

Δtb = M × Kb, Δtf = M × Kf
8.1HW Colligative Properties.doc Colligative Properties of Solvents Use the Equations given in your notes to solve the Colligative Property Questions. ΔTb = m × Kb, ΔTf = m × Kf Freezing Boiling K K Solvent Formula Point f b Point (°C) (°C/m) (°C/m) (°C) Water H2O 0.000 100.000 1.858 0.521 Acetic acid HC2H3O2 16.60 118.5 3.59 3.08 Benzene C6H6 5.455 80.2 5.065 2.61 Camphor C10H16O 179.5 ... 40 ... Carbon disulfide CS2 ... 46.3 ... 2.40 Cyclohexane C6H12 6.55 80.74 20.0 2.79 Ethanol C2H5OH ... 78.3 ... 1.07 1. Which solvent’s freezing point is depressed the most by the addition of a solute? This is determined by the Freezing Point Depression constant, Kf. The substance with the highest value for Kf will be affected the most. This would be Camphor with a constant of 40. 2. Which solvent’s freezing point is depressed the least by the addition of a solute? By the same logic as above, the substance with the lowest value for Kf will be affected the least. This is water. Certainly the case could be made that Carbon disulfide and Ethanol are affected the least as they do not have a constant. 3. Which solvent’s boiling point is elevated the least by the addition of a solute? Water 4. Which solvent’s boiling point is elevated the most by the addition of a solute? Acetic Acid 5. How does Kf relate to Kb? Kf > Kb (fill in the blank) The freezing point constant is always greater. -

Find the Molar Mass of Sodium Carbonate, Na 2CO3. Na 2 X
Moles and Molar Mass The mole is the "counting unit" used by chemists to indicate the number of atoms, ions, molecules, or formula units present in a particular chemical sample. The mole is similar to other counting units that you've used before....pair (2), dozen (12), and gross (144). One mole of a compound contains Avogadro's number (6.022 x 1023) of molecules (molecular compound) or formula units (ionic compound). The molar mass of a compound tells you the mass of 1 mole of that substance. In other words, it tells you the number of grams per mole of a compound. The units for molar mass are, therefore, grams/mole. To find the molar mass of a compound: 1. Use the chemical formula to determine the number of each type of atom present in the compound. 2. Multiply the atomic weight (from the periodic table) of each element by the number of atoms of that element present in the compound. 3. Add it all together and put units of grams/mole after the number. Example: Find the molar mass of sodium carbonate, Na2CO3. Na 2 x 23.0 = 46.0 C 1 x 12.0 = 12.0 O 3 x 16.0 = 48.0 molar = 106.0 g/mole mass For many (but not all) problems, you can simply round the atomic weights and the molar mass to the nearest 0.1 g/mole. HOWEVER, make sure that you use at least as many significant figures in your molar mass as the measurement with the fewest significant figures. -

2 Weighing and Preparation of Aqueous Solutions
2 WEIGHING AND PREPARATION OF AQUEOUS SOLUTIONS Theory In chemistry, a solution is a homogeneous mixture composed of two or more substances. In such a mixture, a solute is dissolved in another substance, known as a solvent. An aqueous solution is a solution in which the solvent is water. Concentration is the measure of how much of a given substance (solute) there is mixed with another substance (solvent). This can apply to any sort of chemical mixture, but most frequently the concept is limited to homogeneous solutions, where it refers to the amount of solute in a solution. For scientific or technical applications, concentration is expressed in a quantitative way. There are a number of different ways to quantitatively express concentration; the most common are listed below. They are based on mass or volume or both. Depending on what they are based on it is not always trivial to convert one measure to the other, because knowledge of the density might be needed to do so. At times this information may not be available, particularly if the temperature varies. Mass can be determined at a precision of ~ 0.1 mg on a routine basis with an analytical balance. Both solids and liquids are easily quantified by weighing. Some units of concentration - particularly the most popular one (molarity) - require to express the mass of substance in the moles. The mol is the SI basic unit that measures an amount of substance. „One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the −1 fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol and is called the Avogadro number.“ The previous definition was based on the number of carbon atoms in 12 g of material and was the following; a mol is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram (or 12 g) of carbon-12 (12C), where the carbon – 12 atoms are unbound, at rest and in their ground state. -

Topic720 Composition: Mole Fraction: Molality: Concentration a Solution Comprises at Least Two Different Chemical Substances
Topic720 Composition: Mole Fraction: Molality: Concentration A solution comprises at least two different chemical substances where at least one substance is in vast molar excess. The term ‘solution’ is used to describe both solids and liquids. Nevertheless the term ‘solution’ in the absence of the word ‘solid’ refers to a liquid. Chemists are particularly expert at identifying the number and chemical formulae of chemical substances present in a given closed system. Here we explore how the chemical composition of a given system is expressed. We consider a simple system prepared using water()l and urea(s) at ambient temperature and pressure. We designate water as chemical substance 1 and urea as chemical substance j, so that the closed system contains an aqueous solution. The amounts of the two substances are given by n1 = = ()wM11 and nj ()wMjj where w1 and wj are masses; M1 and Mj are the molar masses of the two chemical substances. In these terms, n1 and nj are extensive variables. = ⋅ + ⋅ Mass of solution, w n1 M1 n j M j (a) = ⋅ Mass of solvent, w1 n1 M1 (b) = -1 For water, M1 0.018 kg mol . However in reviewing the properties of solutions, chemists prefer intensive composition variables. Mole Fraction The mole fractions of the two substances x1 and xj are given by the following two equations: =+ =+ xnnn111()j xnnnjj()1 j (c) += Here x1 x j 10. In general terms for a system comprising i - chemical substances, the mole fraction of substance k is given by equation (d). ji= = xnkk/ ∑ n j (d) j=1 ji= = Hence ∑ x j 10. -

Avogadro's Constant
Avogadro's Constant Nancy Eisenmenger Question Artem, 8th grade How \Avogadro constant" was invented and how scientists calculated it for the first time? Answer What is Avogadro's Constant? • Avogadros constant, NA: the number of particles in a mole • mole: The number of Carbon-12 atoms in 0.012 kg of Carbon-12 23 −1 • NA = 6:02214129(27) × 10 mol How was Avogadro's Constant Invented? Avogadro's constant was invented because scientists were learning about measuring matter and wanted a way to relate the microscopic to the macroscopic (e.g. how many particles (atoms, molecules, etc.) were in a sample of matter). The first scientists to see a need for Avogadro's constant were studying gases and how they behave under different temperatures and pressures. Amedeo Avogadro did not invent the constant, but he did state that all gases with the same volume, pressure, and temperature contained the same number of gas particles, which turned out to be very important to the development of our understanding of the principles of chemistry and physics. The constant was first calculated by Johann Josef Loschmidt, a German scientist, in 1865. He actually calculated the Loschmidt number, a constant that measures the same thing as Avogadro's number, but in different units (ideal gas particles per cubic meter at 0◦C and 1 atm). When converted to the same units, his number was off by about a factor of 10 from Avogadro's number. That may sound like a lot, but given the tools he had to work with for both theory and experiment and the magnitude of the number (∼ 1023), that is an impressively close estimate. -

Answer Key Chapter 6: Standard Review Worksheet 1
Answer Key Chapter 6: Standard Review Worksheet 1. 1 amu = 1.66 _ 10–24 g. For example, the average atomic mass of sodium is 22.99 amu, which represents the average mass of all the sodium atoms in the world (including all the various isotopes and their relative abundances). So that we will be able to use the mass of a sample of sodium to count the number of atoms of sodium present in the sample, we consider that every sodium atom in a sample has exactly the same mass (the average atomic mass). The average atomic mass of an element is typically not a whole number of amu’s because of the presence of the different isotopes of the element, each with its own relative abundance. Since the relative abundance of an element can be any number, when the weighted average atomic mass of the element is calculated, the average is unlikely to be a whole number. 2. On a microscopic basis, one mole of a substance represents Avogadro’s number (6.022 _ 1023) of individual units (atoms or molecules) of the substance. On a macroscopic basis, one mole of a substance represents the amount of substance present when the molar mass of the substance in grams is taken (for example, 12.01 g of carbon will be one mole of carbon). 3. The molar mass of a compound is the mass in grams of one mole of the compound (6.022 _ 1023 molecules of the compound) and is calculated by summing the average atomic masses of all the atoms present in a molecule of the compound. -

Experiment 9
Experiment Molar Mass by Vapor Density 7 Vapor is the term for a gas produced from the vaporization of a liquid. If it is assumed that the resulting vapor is an ideal gas, then we can use the ideal gas law: By substituting moles for mass and molar mass the following equation is derived after some simple rearrangements: The molar mass of the compound is represented by Mm and d is the vapor density in g per liter. Another way to calculate the molar mass of the sample is to determine the moles of the gas produced. The number of moles can be determined from the measured volume, pressure, ad temperature of the gas. From the mass of the liquid sample used and the moles calculated from experimental measurements, the molar mass of the sample can be determined. Many organic compounds are liquids that vaporized below the boiling point of water. A boiling water bath will be used as a constant temperature (100 C or 373 K) heat source to convert your unknown liquid to a gas. The pressure of the gas will be the barometric pressure of the room and the volume is the volume of the gas inside the container (flask). In this experiment, you will be given an unknown liquid and you will determine the molar mass of the liquid. Equipment and Reagents Analytical balance unknown liquid high capacity balance 200 mL flask tap water thermometer Aluminum foil stand w/ iron ring barometer Pin wire gauze 600 mL beaker buret clamp 1 Procedure 1. On an analytical balance weigh a 200 mL Erlenmeyer flask with a piece of aluminum foil large enough to cover the mouth of the flask. -
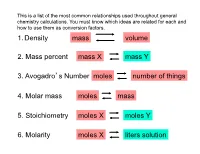
1. Density Mass Volume 3. Avogadro's Number Moles
This is a list of the most common relationships used throughout general chemistry calculations. You must know which ideas are related for each and how to use them as conversion factors. 1. Density mass volume 2. Mass percent mass X mass Y 3. Avogadro’s Number moles number of things 4. Molar mass moles mass 5. Stoichiometry moles X moles Y 6. Molarity moles X liters solution 1. Density mass volume • convert from mass to volume 6.54 g 1 cm3 = 8.32 cm3 = 8.32 mL 0.7857 g • convert from volume to mass 28.56 cm3 0.7857 g = 22.44 g 1 cm3 • calculate density directly density = 371 g = 19.2 g/mL 19.3 cm3 This is a list of the most common relationships used throughout general chemistry calculations. You must know which ideas are related for each and how to use them as conversion factors. 1. Density mass volume 2. Mass percent mass X mass Y 3. Avogadro’s Number moles number of things 4. Molar mass moles mass 5. Stoichiometry moles X moles Y 6. Molarity moles X liters solution 2. Mass percent mass X mass Y 55.5 g CuF2 37.42 g F = 20.8 g F 100 g CuF2 This is a list of the most common relationships used throughout general chemistry calculations. You must know which ideas are related for each and how to use them as conversion factors. 1. Density mass volume 2. Mass percent mass X mass Y 3. Avogadro’s Number moles number of things 4. Molar mass moles mass 5. -

Molar Mass 1 Molar Mass
Molar mass 1 Molar mass In chemistry, the molar mass is a physical property. It is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. The base SI unit for molar mass is kg/mol. However, for historical reasons, molar masses are almost always expressed in g/mol. As an example, the molar mass of water is approximately: M(H O) ≈ 18 g⋅mol−1 2 Molar masses of elements The molar mass of atoms of an element is given by the atomic mass of the element multiplied by the molar mass constant, M −3 u = 1×10 kg/mol = 1 g/mol: M(H) = 1.007 97(7) × 1 g/mol = 1.007 97(7) g/mol M(S) = 32.065(5) × 1 g/mol = 32.065(5) g/mol M(Cl) = 35.453(2) × 1 g/mol = 35.453(2) g/mol M(Fe) = 55.845(2) × 1 g/mol = 55.845(2) g/mol. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole). Some elements are usually encountered as molecules, e.g. hydrogen (H 2), sulfur (S 8), chlorine (Cl 2). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule: M(H 2) = 2 × 1.007 97(7) × 1 g/mol = 2.015 88(14) g/mol M(S 8) = 8 × 32.065(5) × 1 g/mol = 256.52(4) g/mol M(Cl 2) = 2 × 35.453(2) × 1 g/mol = 70.906(4) g/mol.