Nuclear Criticality Safety Engineer Training Module 1 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NRP-3 the Effect of Beryllium Interaction with Fast Neutrons on the Reactivity of Etrr-2 Research Reactor
Seventh Conference of Nuclear Sciences & Applications 6-10 February 2000, Cairo, Egypt NRP-3 The Effect Of Beryllium Interaction With Fast Neutrons On the Reactivity Of Etrr-2 Research Reactor Moustafa Aziz and A.M. EL Messiry National Center for Nuclear Safety Atomic Energy Autho , Cairo , Egypt ABSTRACT The effect of beryllium interactions with fast neutrons is studied for Etrr_2 research reactors. Isotope build up inside beryllium blocks is calculated under different irradiation times. A new model for the Etrr_2 research reactor is designed using MCNP code to calculate the reactivity and flux change of the reactor due to beryllium poison. Key words: Research Reactors , Neutron Flux , Beryllium Blocks, Fast Neutrons and Reactivity INTRODUCTION Beryllium irradiated by fast neutrons with energies in the range 0.7-20 Mev undergoes (n,a) and (n,2n) reactions resulting in subsequent formation of the isotopes lithium (Li-6), tritium (H-3) and helium (He-3 and He-4 ). Beryllium interacts with fast neutrons to produce 6He that decay 6 6 ( T!/2 =0.8 s) to produce Li . Li interacts with neutron to produce tritium which suffer /? (T1/2 = 12.35 year) decay and converts to 3He which finally interact with neutron to produce tritium. These processes some times defined as Beryllium poison. Negative effect of this process are met whenever beryllium is used in a thermal reactor as a reflector or moderator . Because of their large thermal neutron absorption cross sections , the buildup of He-3 and Li-6 concentrations ,initiated by the Be(n,a) reaction , results in large negative reactivities which alter the reactivity , flux and power distributions. -

Hadron Collider Physics
KEK-PH Lectures and Workshops Hadron Collider Physics Zhen Liu University of Maryland 08/05/2020 Part I: Basics Part II: Advanced Topics Focus Collider Physics is a vast topic, one of the most systematically explored areas in particle physics, concerning many observational aspects in the microscopic world • Focus on important hadron collider concepts and representative examples • Details can be studied later when encounter References: Focus on basic pictures Barger & Philips, Collider Physics Pros: help build intuition Tao Han, TASI lecture, hep-ph/0508097 Tilman Plehn, TASI lecture, 0910.4182 Pros: easy to understand Maxim Perelstein, TASI lecture, 1002.0274 Cons: devils in the details Particle Data Group (PDG) and lots of good lectures (with details) from CTEQ summer schools Zhen Liu Hadron Collider Physics (lecture) KEK 2020 2 Part I: Basics The Large Hadron Collider Lyndon R Evans DOI:10.1098/rsta.2011.0453 Path to discovery 1995 1969 1974 1969 1979 1969 1800-1900 1977 2012 2000 1975 1983 1983 1937 1962 Electric field to accelerate 1897 1956 charged particles Synchrotron radiation 4 Zhen Liu Hadron Collider Physics (lecture) KEK 2020 4 Zhen Liu Hadron Collider Physics (lecture) KEK 2020 5 Why study (hadron) colliders (now)? • Leading tool in probing microscopic structure of nature • history of discovery • Currently running LHC • Great path forward • Precision QFT including strong dynamics and weakly coupled theories • Application to other physics probes • Set-up the basic knowledge to build other subfield of elementary particle physics Zhen Liu Hadron Collider Physics (lecture) KEK 2020 6 Basics: Experiment & Theory Zhen Liu Hadron Collider Physics (lecture) KEK 2020 7 Basics: How to make measurements? Zhen Liu Hadron Collider Physics (lecture) KEK 2020 8 Part I: Basics Basic Parameters Basics: Smashing Protons & Quick Estimates Proton Size ( ) Proton-Proton cross section ( ) Particle Physicists use the unit “Barn”2 1 = 100 The American idiom "couldn't hit the broad side of a barn" refers to someone whose aim is very bad. -

HEPAP Looks Into the Future
Volume 20 Friday, August 29, 1997 Number 17 f INSIDE HEPAP Looks into 2 HEPAP: Voice of the Community the Future 5 Profiles in HEPAP subpanel meets at Fermilab to chart the future of Particle Physics: high-energy physics in the U.S. Dave McGinnis by Donald Sena and Sharon Butler, Office of Public Affairs 6 Barns of Fermilab Charged with recommending how best to In a letter to HEPAP, Martha Krebs, position the U.S. particle physics community Director of the U.S. Department of Energy’s for new facilities beyond CERN’s Large Office of Energy Research, directed the Hadron Collider, a subpanel of the High- subpanel to “recommend a scenario for an Energy Physics Advisory Panel met at Fermilab optimal and balanced U.S. high-energy physics August 14-16 to hear presentations on such program over the next decade,” with “new topics as the research agenda for Fermilab’s facilities to address physics opportunities Run II, the complicated upgrades to the CDF beyond the LHC.” She asked the subpanel and DZero detectors and research on future to consider a future course in light of three accelerators. continued on page 3 Photo by Reidar Hahn Dixon Bogert, deputy project manager for the Main Injector, leads a tour for HEPAP subpanel members and DOE officials. HEPAP: Voice of the Community by Donald Sena and Sharon Butler, Office of Public Affairs The High-Energy Physics Advisory In 1983, for example, a HEPAP According to Fermilab physicist Cathy Panel traces its history to the 1960s, subpanel recommended terminating Newman-Holmes, outgoing member of when -

Searches for a Charged Higgs Boson in ATLAS and Development of Novel Technology for Future Particle Detector Systems
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1222 Searches for a Charged Higgs Boson in ATLAS and Development of Novel Technology for Future Particle Detector Systems DANIEL PELIKAN ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-554-9153-6 UPPSALA urn:nbn:se:uu:diva-242491 2015 Dissertation presented at Uppsala University to be publicly examined in Polhemssalen, Ångströmlaboratoriet, Lägerhyddsvägen 1, Uppsala, Friday, 20 March 2015 at 10:00 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Prof. Dr. Fabrizio Palla (Istituto Nazionale di Fisica Nucleare (INFN) Pisa). Abstract Pelikan, D. 2015. Searches for a Charged Higgs Boson in ATLAS and Development of Novel Technology for Future Particle Detector Systems. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1222. 119 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-554-9153-6. The discovery of a charged Higgs boson (H±) would be a clear indication for physics beyond the Standard Model. This thesis describes searches for charged Higgs bosons with the ATLAS experiment at CERN’s Large Hadron Collider (LHC). The first data collected during the LHC Run 1 is analysed, searching for a light charged Higgs boson (mH±<mtop), which decays predominantly into a tau-lepton and a neutrino. Different final states with one or two leptons (electrons or muons), as well as leptonically or hadronically decaying taus, are studied, and exclusion limits are set. The background arising from misidentified non-prompt electrons and muons was estimated from data. This so-called "Matrix Method'' exploits the difference in the lepton identification between real, prompt, and misidentified or non-prompt electrons and muons. -
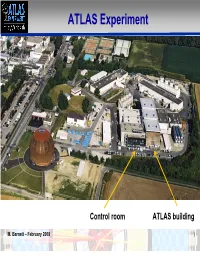
ATLAS Experiment
ATLAS Experiment Control room ATLAS building M. Barnett – February 2008 1 Final piece of ATLAS lowered last Friday (the second small muon wheel) M. Barnett – February 2008 2 M. Barnett – February 2008 3 Still to be done Connecting: Cables, Fibers, Cryogenics M. Barnett – February 2008 4 Celebrations M. Barnett – February 2008 5 News Coverage News media are flooding CERN well in advance of startup "Particle physics is the unbelievable in pursuit of the unimaginable. To pinpoint the smallest fragments of the universe you have to build the biggest machine in the world. To recreate the first millionths of a second of creation you have to focus energy on an awesome scale." The Guardian M. Barnett – February 2008 6 Three full pages in New York Times M. Barnett – February 2008 7 New York Times M. Barnett – February 2008 8 National Geographic Magazine M. Barnett – February 2008 9 National Geographic Magazine M. Barnett – February 2008 10 National Geographic Magazine M. Barnett – February 2008 11 M. Barnett – February 2008 12 M. Barnett – February 2008 13 M. Barnett – February 2008 14 M. Barnett – February 2008 15 M. Barnett – February 2008 16 M. Barnett – February 2008 17 M. Barnett – February 2008 18 M. Barnett – February 2008 19 M. Barnett – February 2008 20 M. Barnett – February 2008 21 M. Barnett – February 2008 22 And on television (shortened version) M. Barnett – February 2008 23 An ATLAS expert explains the Higgs evidence to a layperson. M. Barnett – February 2008 24 US-LHC Student Journalism Program April 2-7, 2008 (overlapping Open Days) Six teams of high school students (3 students/1 teacher) are going to CERN to report on the LHC startup. -

How and Why to Go Beyond the Discovery of the Higgs Boson
How and Why to go Beyond the Discovery of the Higgs Boson John Alison University of Chicago http://hep.uchicago.edu/~johnda/ComptonLectures.html Lecture Outline April 1st: Newton’s dream & 20th Century Revolution April 8th: Mission Barely Possible: QM + SR April 15th: The Standard Model April 22nd: Importance of the Higgs April 29th: Guest Lecture May 6th: The Cannon and the Camera May 13th: The Discovery of the Higgs Boson May 20th: Problems with the Standard Model May 27th: Memorial Day: No Lecture June 3rd: Going beyond the Higgs: What comes next ? 2 Reminder: The Standard Model Description fundamental constituents of Universe and their interactions Triumph of the 20th century Quantum Field Theory: Combines principles of Q.M. & Relativity Constituents (Matter Particles) Spin = 1/2 Leptons: Quarks: νe νµ ντ u c t ( e ) ( µ) ( τ ) ( d ) ( s ) (b ) Interactions Dictated by principles of symmetry Spin = 1 QFT ⇒ Particle associated w/each interaction (Force Carriers) γ W Z g Consistent theory of electromagnetic, weak and strong forces ... ... provided massless Matter and Force Carriers Serious problem: matter and W, Z carriers have Mass ! 3 Last Time: The Higgs Feild New field (Higgs Field) added to the theory Allows massive particles while preserve mathematical consistency Works using trick: “Spontaneously Symmetry Breaking” Zero Field value Symmetric in Potential Energy not minimum Field value of Higgs Field Ground State 0 Higgs Field Value Ground state (vacuum of Universe) filled will Higgs field Leads to particle masses: Energy cost to displace Higgs Field / E=mc 2 Additional particle predicted by the theory. Higgs boson: H Spin = 0 4 Last Time: The Higgs Boson What do we know about the Higgs Particle: A Lot Higgs is excitations of v-condensate ⇒ Couples to matter / W/Z just like v X matter: e µ τ / quarks W/Z h h ~ (mass of matter) ~ (mass of W or Z) matter W/Z Spin: 0 1/2 1 3/2 2 Only thing we don’t (didn’t!) know is the value of mH 5 History of Prediction and Discovery Late 60s: Standard Model takes modern form. -

Atomic Weights and Isotopic Abundances*
Pure&App/. Chem., Vol. 64, No. 10, pp. 1535-1543, 1992. Printed in Great Britain. @ 1992 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY INORGANIC CHEMISTRY DIVISION COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCES* 'ATOMIC WEIGHT' -THE NAME, ITS HISTORY, DEFINITION, AND UNITS Prepared for publication by P. DE BIEVRE' and H. S. PEISER2 'Central Bureau for Nuclear Measurements (CBNM), Commission of the European Communities-JRC, B-2440 Geel, Belgium 2638 Blossom Drive, Rockville, MD 20850, USA *Membership of the Commission for the period 1989-1991 was as follows: J. R. De Laeter (Australia, Chairman); K. G. Heumann (FRG, Secretary); R. C. Barber (Canada, Associate); J. CCsario (France, Titular); T. B. Coplen (USA, Titular); H. J. Dietze (FRG, Associate); J. W. Gramlich (USA, Associate); H. S. Hertz (USA, Associate); H. R. Krouse (Canada, Titular); A. Lamberty (Belgium, Associate); T. J. Murphy (USA, Associate); K. J. R. Rosman (Australia, Titular); M. P. Seyfried (FRG, Associate); M. Shima (Japan, Titular); K. Wade (UK, Associate); P. De Bi&vre(Belgium, National Representative); N. N. Greenwood (UK, National Representative); H. S. Peiser (USA, National Representative); N. K. Rao (India, National Representative). Republication of this report is permitted without the need for formal IUPAC permission on condition that an acknowledgement, with full reference together with IUPAC copyright symbol (01992 IUPAC), is printed. Publication of a translation into another language is subject to the additional condition of prior approval from the relevant IUPAC National Adhering Organization. ’Atomic weight‘: The name, its history, definition, and units Abstract-The widely used term “atomic weight” and its acceptance within the international system for measurements has been the subject of debate. -

Answer Key Chapter 6: Standard Review Worksheet 1
Answer Key Chapter 6: Standard Review Worksheet 1. 1 amu = 1.66 _ 10–24 g. For example, the average atomic mass of sodium is 22.99 amu, which represents the average mass of all the sodium atoms in the world (including all the various isotopes and their relative abundances). So that we will be able to use the mass of a sample of sodium to count the number of atoms of sodium present in the sample, we consider that every sodium atom in a sample has exactly the same mass (the average atomic mass). The average atomic mass of an element is typically not a whole number of amu’s because of the presence of the different isotopes of the element, each with its own relative abundance. Since the relative abundance of an element can be any number, when the weighted average atomic mass of the element is calculated, the average is unlikely to be a whole number. 2. On a microscopic basis, one mole of a substance represents Avogadro’s number (6.022 _ 1023) of individual units (atoms or molecules) of the substance. On a macroscopic basis, one mole of a substance represents the amount of substance present when the molar mass of the substance in grams is taken (for example, 12.01 g of carbon will be one mole of carbon). 3. The molar mass of a compound is the mass in grams of one mole of the compound (6.022 _ 1023 molecules of the compound) and is calculated by summing the average atomic masses of all the atoms present in a molecule of the compound. -

5.1: Atomic Mass Unit 5.1: Atomic Mass
Topic 5: Stoichiometry - Chemical Arithmetic Masses of some atoms: 1 -24 16 -23 1H = 1.6736×10 g 8 O = 2.6788 ×10 g 238 -22 92U = 3.9851×10 g Introducing…….the Atomic Mass Unit (amu) 1 amu = 1.66054 x 10-24 g 1 5.1: Atomic Mass Unit Atomic Mass is defined relative to Carbon -12 isotope 12 12 amu is the mass of the 6 C isotope of carbon Carbon -12 atom = 12.000 amu Hydrogen -1 atom = 1.008 amu Oxygen -16 atom = 15.995 amu Chlorine -35 atom = 34.969 amu 2 5.1: Atomic Mass - Natural Abundance We deal with the naturally occurring mix of isotopes, rather than pure isotopes Carbon has three natural isotopes Isotope Mass (amu) Abundance (%) 12C 12.000 98.892 13C 13.00335 1.108 14C 14.00317 1 x 10-4 Any shovelful of Carbon from living material will have a Naturally Occurring Abundance of 98.892% 12C, 1.108% 13C and 0.0001% 14C 3 1 5.1: Atomic Mass - Relative Abundance How do we take into account the naturally occurring Abundances? Take the average mass of the various isotopes weighted according to their Relative Abundances Relative Isotope Mass (amu) Abundance (%) Abundance 12C 12.000 98.892 0.98892 13C 13.00335 1.108 0.0108 -6 14C 14.00317 1 x 10-4 1 x 10 N.B. The % Abundance adds up to 100 The Relative Abundance adds up to 1 4 5.1: Average Atomic Mass Relative Isotope Mass (amu) Abundance (%) Abundance 12C 12.000 98.892 0.98892 13C 13.00335 1.108 0.0108 -6 14C 14.00317 1 x 10-4 1 x 10 The Average Atomic Mass is given by: (0.98892 x 12.000 amu) + (0.01108 x 13.00335 amu) + (1 x 10-6 x 14.00317 amu) = 12.011 amu 5 5.1: Atomic and Molecular Mass You can calculate the mass of any compound from the sum of the atomic masses from the periodic table. -

Make an Atom Vocabulary Grade Levels
MAKE AN ATOM Fundamental to physical science is a basic understanding of the atom. Atoms are comprised of protons, neutrons, and electrons. Protons and neutrons are at the center of the atom while electrons live in lobe-shaped clouds outside the nucleus. The number of electrons usually matches the number of protons, yielding a net neutral charge for the atom. Sometimes an atom has less neutrons or more neutrons than protons. This is called an isotope. If an atom has different numbers of electrons than protons, then it is an ion. If an atom has different numbers of protons, it is a different element all together. Scientists at Idaho National Laboratory study, create, and use radioactive isotopes like Uranium 234. The 234 means this isotope has an atomic mass of 234 Atomic Mass Units (AMU). GRADE LEVELS: 3-8 VOCABULARY Atom – The basic unit of a chemical element. Proton – A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron, but of opposite sign. Neutron – A subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. Electron – A stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids. Orbital – Each of the actual or potential patterns of electron density that may be formed on an atom or molecule by one or more electrons. Ion – An atom or molecule with a net electric charge due to the loss or gain of one or more electrons. -

Long-Range Beam–Beam Effects in the Tevatron* V
Published by CERN in the Proceedings of the ICFA Mini-Workshop on Beam–Beam Effects in Hadron Colliders, CERN, Geneva, Switzerland, 18–22 March 2013, edited by W. Herr and G. Papotti, CERN–2014–004 (CERN, Geneva, 2014) LONG-RANGE BEAM–BEAM EFFECTS IN THE TEVATRON* V. Shiltsev#, A.Valishev, FNAL, Batavia, IL, USA Abstract Long-range beam–beam effects occurred in the Tevatron at all stages (injection, ramp, squeeze, and collisions) and affected both proton and antiproton beams. They resulted in beam losses and emittance blow-ups, which occurred in remarkable bunch-to-bunch dependent patterns. On the way to record-high luminosities of the collider, many issues related to the long-range beam– beam interactions have been addressed. Below we present a short overview of the long-range beam–beam effects in the Tevatron. (For a detailed discussion on the beam– beam effects in the Tevatron please see reviews in Refs. [1–3] and references therein). HELICAL ORBITS IN TEVAT R O N Beam–beam interactions in the Tevatron differ between the injection and collision stages. The helical orbits were introduced to provide sufficient separation between the proton and antiproton beams in order to reduce Figure 2: The pattern of the Tevatron helical orbits at the detrimental beam–beam effects, e.g. tune shifts, coupling, collision stage. and high-order resonance driving terms. In 36 × 36 bunch Initially, there were six separator groups (three operation, each bunch experienced 72 long-range horizontal and three vertical) in the arcs between the two interactions per revolution at injection, but at collision main interaction points, B0 (CDF) and D0. -

Molar Mass 1 Molar Mass
Molar mass 1 Molar mass In chemistry, the molar mass is a physical property. It is defined as the mass of a given substance (chemical element or chemical compound) divided by its amount of substance. The base SI unit for molar mass is kg/mol. However, for historical reasons, molar masses are almost always expressed in g/mol. As an example, the molar mass of water is approximately: M(H O) ≈ 18 g⋅mol−1 2 Molar masses of elements The molar mass of atoms of an element is given by the atomic mass of the element multiplied by the molar mass constant, M −3 u = 1×10 kg/mol = 1 g/mol: M(H) = 1.007 97(7) × 1 g/mol = 1.007 97(7) g/mol M(S) = 32.065(5) × 1 g/mol = 32.065(5) g/mol M(Cl) = 35.453(2) × 1 g/mol = 35.453(2) g/mol M(Fe) = 55.845(2) × 1 g/mol = 55.845(2) g/mol. Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole). Some elements are usually encountered as molecules, e.g. hydrogen (H 2), sulfur (S 8), chlorine (Cl 2). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule: M(H 2) = 2 × 1.007 97(7) × 1 g/mol = 2.015 88(14) g/mol M(S 8) = 8 × 32.065(5) × 1 g/mol = 256.52(4) g/mol M(Cl 2) = 2 × 35.453(2) × 1 g/mol = 70.906(4) g/mol.