Architectonic Identification of the Core Region in Auditory Cortex Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -
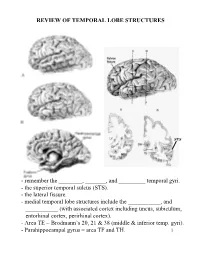
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

In the Motion Area of the Superior Temporal Sulcus Were Directionally Selective. 5
J. Phyeiol. (1978), 277, pp. 273-290 273 With 1 plate and 10 text-figurem Printed in Great Britain UNIFORMITY AND DIVERSITY OF STRUCTURE AND FUNCTION IN RHESUS MONKEY PRESTRIATE VISUAL CORTEX BY S. M. ZEKI* From the Department of Anatomy, University College London, London WC1E 6BT (Received 31 March 1977) SUMMARY 1. Recordings were made from single neurones, or small clusters of cells, in five prestriate visual areas of rhesus monkey cortex. The cells were studied for their binocularity, as well as for their orientational, motion and colour preferences. In all, 1500 cells were studied, 250 cells for each of the areas V2, V3, V3A and the motion area ofthe posterior bank ofthe superior temporal sulcus, and 500 cells for V4. All the cells referred to in this study can be placed in one prestriate area or another unambiguously. 2. The great majority of cells in all areas were binocularly driven, without mono- cular preferences. Within each area, there were cells that either preferred binocular stimulation markedly, or were responsive to binocular stimulation only. The ocular interaction histograms for all areas are remarkably similar when tested at a fixed disparity. 3. Over 70 % of the cells in areas V2, V3 and V3A were selective for orientation. The receptive fields of cells were larger in V3 and V3A than in V2. By contrast, less than 50 % of the cells in V4 and the motion area of the superior temporal sulcus were orientation selective. 4. Directionally selective cells were found in all areas. But they were present in small numbers (< 15 %) in areas V2, V3, V3A and V4. -

Supporting Information for “Endocast Morphology of Homo Naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, S
Supporting Information for “Endocast Morphology of Homo naledi from the Dinaledi Chamber, South Africa” Ralph L. Holloway, Shawn D. Hurst, Heather M. Garvin, P. Thomas Schoenemann, William B. Vanti, Lee R. Berger, and John Hawks What follows are our descriptions, illustrations, some basic interpretation, and a more specific discussion of the functional, comparative, and taxonomic issues surrounding these hominins. We use the neuroanatomical nomenclature from Duvernoy (19). DH1 Occipital The DH1 occipital fragment (Figs 1, S2) measures ca 105 mm in width between left temporo- occipital incisure and right sigmoid sinus. It is 61 mm in height on the left side, and 47 mm on the right side. The fragment covers the entire left and mostly complete right occipital lobes. The lobes are strongly asymmetrical, with the left clearly larger than the right, and more posteriorly protruding. There are faint traces of a lateral remnant of the lunate sulcus on the left side, and a dorsal bounding lunate as well (#4 and #6 in Fig 1). The right side shows a very small groove at the end of the lateral sinus, which could be a remnant of the lunate sulcus. The major flow from the longitudinal sinus is to the right. Small portions of both cerebellar lobes, roughly 15 mm in height are present. There is a suggestion of a great cerebellar sulcus on the right side. The width from the left lateral lunate impression to the midline is 43mm. The distance from left occipital pole (the most posteriorly projecting point, based on our best estimate of the proper orientation) to the mid-sagittal plane is 30 mm. -

Surgical Anatomy and Techniques
SURGICAL ANATOMY AND TECHNIQUES MICROSURGICAL APPROACHES TO THE MEDIAL TEMPORAL REGION:AN ANATOMICAL STUDY Alvaro Campero, M.D. OBJECTIVE: To describe the surgical anatomy of the anterior, middle, and posterior Department of Neurological Surgery, portions of the medial temporal region and to present an anatomic-based classification University of Florida, of the approaches to this area. Gainesville, Florida METHODS: Twenty formalin-fixed, adult cadaveric specimens were studied. Ten brains Gustavo Tro´ccoli, M.D. provided measurements to compare different surgical strategies. Approaches were demon- Department of Neurological Surgery, strated using 10 silicon-injected cadaveric heads. Surgical cases were used to illustrate the Hospital “Dr. J. Penna,” results by the different approaches. Transverse lines at the level of the inferior choroidal point Bahı´a Blanca, Argentina and quadrigeminal plate were used to divide the medial temporal region into anterior, middle, and posterior portions. Surgical approaches to the medial temporal region were classified into Carolina Martins, M.D. four groups: superior, lateral, basal, and medial, based on the surface of the lobe through which Department of Neurological Surgery, University of Florida, the approach was directed. The approaches through the medial group were subdivided further Gainesville, Florida into an anterior approach, the transsylvian transcisternal approach, and two posterior ap- proaches, the occipital interhemispheric and supracerebellar transtentorial approaches. Juan C. Fernandez-Miranda, M.D. RESULTS: The anterior portion of the medial temporal region can be reached through Department of Neurological Surgery, University of Florida, the superior, lateral, and basal surfaces of the lobe and the anterior variant of the Gainesville, Florida approach through the medial surface. -

The Depth Asymmetry of Superior Temporal Sulcus
New human-specific brain landmark: The depth asymmetry of superior temporal sulcus François Leroya,1, Qing Caib, Stephanie L. Bogartc, Jessica Duboisa, Olivier Coulond, Karla Monzalvoa, Clara Fischere, Hervé Glasela, Lise Van der Haegenf, Audrey Bénézita, Ching-Po Ling, David N. Kennedyh, Aya S. Iharai, Lucie Hertz-Pannierj, Marie-Laure Moutardk, Cyril Pouponl, Marc Brysbaerte, Neil Robertsm, William D. Hopkinsc, Jean-François Mangine, and Ghislaine Dehaene-Lambertza aCognitive Neuroimaging Unit, U992, eAnalysis and Processing of Information Unit, jClinical and Translational Applications Research Unit, U663, and lNuclear Magnetic Resonance Imaging and Spectroscopy Unit, Office of Atomic Energy and Alternative Energies (CEA), INSERM, NeuroSpin, Gif-sur-Yvette 91191, France; bShanghai Key Laboratory of Brain Functional Genomics, East China Normal University, Shanghai 200241, China; cDivision of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, GA 30322; dLaboratory for Systems and Information Science, UMR CNRS 7296, Aix-Marseille University, Marseille 13284, France; fDepartment of Experimental Psychology, Ghent University, Ghent B-9000, Belgium; gInstitute of Neuroscience, National Yang-Ming University, Taipei City 112, Taiwan; hCenter for Morphometric Analysis, Neuroscience Center, Massachusetts General Hospital, Boston, MA 02114; iCenter for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka 565-0871 Japan; kNeuropediatrics Department, Trousseau -

Pars Triangularis Asymmetry and Language Dominance ANNE L
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 719-722, January 1996 Neurobiology Pars triangularis asymmetry and language dominance ANNE L. FOUNDAS*, CHRISTIANA M. LEONARDO§, ROBIN L. GILMOREt¶, EILEEN B. FENNELLtII, AND KENNETH M. HEILMAN4:¶** *Department of Psychiatry and Neurology, Tulane University School of Medicine and Neurology Service, Veterans Affairs Medical Center, 1430 Tulane Avenue, New Orleans, LA 70112-2632; and *Center for Neuropsychological Studies, Departments of §Neuroscience and 1Neurology and I'Clinical and Health Psychology, University of Florida College of Medicine and **Neurology Service, Veterans Affairs Medical Center, Gainesville, FL 32608-1197 Communicated by Philip Teitelbaum, University of Florida, Gainesville, FL, October 10, 1995 (received for review March 15, 1995) ABSTRACT The pars triangularis is a portion of Broca's planum temporale can be performed (19-22). Given these area. The convolutions that form the inferior and caudal advances in MRI technology, reliable measures of language extent of the pars triangularis include the anterior horizontal laterality and handedness can be evaluated in the same indi- and anterior ascending rami of the sylvian fissure, respec- viduals, thus allowing direct structure-function correlations. tively. To learn if there are anatomic asymmetries of the pars Using this technique, Steinmetz et al. (22) studied planum triangularis, these convolutions were measured on volumetric temporale asymmetries in a group of left- and right-handers. magnetic resonance imaging scans of 11 patients who had A leftward asymmetry of the planum temporale was docu- undergone selective hemispheric anesthesia (Wada testing) to mented with a greater degree of leftward asymmetry in the determine hemispheric speech and language lateralization. -

A Volumetric Comparison of the Insular Cortex and Its Subregions in Primates
Journal of Human Evolution 64 (2013) 263e279 Contents lists available at SciVerse ScienceDirect Journal of Human Evolution journal homepage: www.elsevier.com/locate/jhevol A volumetric comparison of the insular cortex and its subregions in primates Amy L. Bauernfeind a,b,*, Alexandra A. de Sousa c, Tanvi Avasthi b, Seth D. Dobson d, Mary Ann Raghanti e, Albert H. Lewandowski f, Karl Zilles g,h, Katerina Semendeferi i, John M. Allman j, Arthur D. (Bud) Craig k, Patrick R. Hof l,m, Chet C. Sherwood b a Department of Anthropology, Hominid Paleobiology Doctoral Program, The George Washington University, Washington, DC 20052, USA b Department of Anthropology, The George Washington University, Washington, DC 20052, USA c Department of Life Sciences, Forensic Sciences Center, University of Coimbra, 3001-401 Coimbra, Portugal d Department of Anthropology, Dartmouth College, Dartmouth, NH 03755, USA e Department of Anthropology and School of Biomedical Sciences, Kent State University, Kent, OH 44242, USA f Cleveland Metroparks Zoo, Cleveland, OH 44109, USA g Institute of Neuroscience and Medicine INM-1, Research Center Jülich, D-52525 Jülich, Germany h C. and O. Vogt Institute of Brain Research, Heinrich Heine University, Düsseldorf, D-40225 Düsseldorf, Germany i Department of Anthropology, University of California San Diego, La Jolla, CA 92093, USA j Division of Biology, California Institute of Technology, Pasadena, CA 91125, USA k Atkinson Research Laboratory, Barrow Neurological Institute, Phoenix, AZ 85041, USA l Fishberg Department of Neuroscience and Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA m New York Consortium in Evolutionary Primatology, New York, USA article info abstract Article history: The neuronal composition of the insula in primates displays a gradient, transitioning from granular Received 4 April 2012 neocortex in the posterior-dorsal insula to agranular neocortex in the anterior-ventral insula with an in- Accepted 31 December 2012 termediate zone of dysgranularity. -

Sex-Specific Differences in Resting-State Functional Connectivity
www.nature.com/scientificreports OPEN Sex‑specifc diferences in resting‑state functional connectivity of large‑scale networks in postconcussion syndrome Reema Shaf1,2*, Adrian P. Crawley3,4, Maria Carmela Tartaglia5,6,7,8, Charles H. Tator4,6,7,9,10, Robin E. Green1,2,3,4,6, David J. Mikulis3,4,6 & Angela Colantonio1,2,11 Concussions are associated with a range of cognitive, neuropsychological and behavioral sequelae that, at times, persist beyond typical recovery times and are referred to as postconcussion syndrome (PCS). There is growing support that concussion can disrupt network‑based connectivity post‑injury. To date, a signifcant knowledge gap remains regarding the sex‑specifc impact of concussion on resting state functional connectivity (rs‑FC). The aims of this study were to (1) investigate the injury‑ based rs‑FC diferences across three large‑scale neural networks and (2) explore the sex‑specifc impact of injury on network‑based connectivity. MRI data was collected from a sample of 80 concussed participants who fulflled the criteria for postconcussion syndrome and 31 control participants who did not have any history of concussion. Connectivity maps between network nodes and brain regions were used to assess connectivity using the Functional Connectivity (CONN) toolbox. Network based statistics showed that concussed participants were signifcantly diferent from healthy controls across both salience and fronto‑parietal network nodes. More specifcally, distinct subnetwork components were identifed in the concussed sample, with hyperconnected frontal nodes and hypoconnected posterior nodes across both the salience and fronto‑parietal networks, when compared to the healthy controls. Node‑to‑region analyses showed sex‑specifc diferences across association cortices, however, driven by distinct networks. -

'Absolute Pitch—Functional Evidence of Speech-Relevant Auditory Acuity'
Oechslin, M; Meyer, M; Jäncke, L (2010). Absolute Pitch—Functional Evidence of Speech-Relevant Auditory Acuity. Cordical Cortex, 20(2):447-455. Postprint available at: http://www.zora.uzh.ch University of Zurich Posted at the Zurich Open Repository and Archive, University of Zurich. Zurich Open Repository and Archive http://www.zora.uzh.ch Originally published at: Cordical Cortex 2010, 20(2):447-455. Winterthurerstr. 190 CH-8057 Zurich http://www.zora.uzh.ch Year: 2010 Absolute Pitch—Functional Evidence of Speech-Relevant Auditory Acuity Oechslin, M; Meyer, M; Jäncke, L Oechslin, M; Meyer, M; Jäncke, L (2010). Absolute Pitch—Functional Evidence of Speech-Relevant Auditory Acuity. Cordical Cortex, 20(2):447-455. Postprint available at: http://www.zora.uzh.ch Posted at the Zurich Open Repository and Archive, University of Zurich. http://www.zora.uzh.ch Originally published at: Cordical Cortex 2010, 20(2):447-455. Cerebral Cortex February 2010;20:447--455 doi:10.1093/cercor/bhp113 Advance Access publication July 10, 2009 Absolute Pitch—Functional Evidence of Mathias S. Oechslin, Martin Meyer and Lutz Ja¨ncke Speech-Relevant Auditory Acuity Department of Neuropsychology, University of Zurich, CH-8050 Zurich, Switzerland Absolute pitch (AP) has been shown to be associated with Interestingly, memorization of musical pieces without referring morphological changes and neurophysiological adaptations in the to a notation is an important key issue of this approach which planum temporale, a cortical area involved in higher-order auditory particularly puts emphasis on auditory features, namely and speech perception processes. The direct link between speech discrimination and representation of pitch and timbre. -

Cross-Species Comparisons of the Retrosplenial Cortex in Primates: Through Time and Neuropil Space
! ! ! CROSS-SPECIES COMPARISONS OF THE RETROSPLENIAL CORTEX IN PRIMATES: THROUGH TIME AND NEUROPIL SPACE A thesis submitted to Kent State University in partial fulfillment of the requirement for the degree of Master of Arts by Mitch Sumner May, 2013 Thesis written by Mitch Andrew Sumner B.A., Indiana University of Pennsylvania, USA 2009 Approved by: __________________________________________ Dr. Mary Ann Raghanti Advisor __________________________________________ Dr. Richard Meindl Chair, Department of Anthropology __________________________________________ Dr. Raymond A. Craig Associate Dean, Collage of Arts and Sciences ! ii! TABLE OF CONTENTS LIST OF FIGURES ................................................................................................. v LIST OF TABLES ................................................................................................. vi AKNOWLEDGEMENTS ..................................................................................... vii ABSTRACT ......................................................................................................... viii Chapter I. INTRODUCTION ............................................................................. 1 Declarative vs. nondeclarative memory ........................................... 4 Episodic memory and mental time travel in humans ....................... 6 Memory in non-human animals ........................................................ 9 Connectivity and behavior .............................................................. 13 Neuropil