Biosynthesis of Major Histocompatibility Complex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 ICAM3-Fc Outperforms Receptor-Specific Antibodies
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 10 April 2019 doi:10.20944/preprints201904.0118.v1 Peer-reviewed version available at Molecules 2019, 24, 1825; doi:10.3390/molecules24091825 ICAM3-Fc outperforms receptor-specific antibodies targeted nanoparticles to dendritic cells for cross-presentation Luis J. Cruz,1* Paul J. Tacken,2 Johan S. van der Schoot,2 Felix Rueda,3 Ruurd Torensma,2 Carl G. Figdor2* 1Translational Nanobiomaterials and Imaging, Department of Radiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands. 2Department of Tumor Immunology, Radboud Insititute for Molecular Life Sciences, Radboud University Medical Center, Postbox 9101, 6500 HB Nijmegen, The Netherlands. 3Department of Biochemistry and Molecular Biology, University of Barcelona, Diagonal 643, 08028 Barcelona, Spain. Running title: ICAM3-Fc- versus antibody-targeted NP vaccines to human DCs Keywords: Delivery system; nanoparticle; targeting. AUTHOR INFORMATION Corresponding Author *Dr. Luis J. Cruz Translational Nanobiomaterials and Imaging, Department of Radiology, Bldg.1, C5-60. Leiden University Medical Center, Albinusdreef 2 2333 ZA Leiden, The Netherlands Tel: +31 71 5263025 Email: [email protected] *Prof. Dr. Carl G. Figdor Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Postbox 9101, 6500 HB Nijmegen, The Netherlands. Fax: +31-24-3540339; Tel.: +31-24-3617600; E-mail: [email protected] 1 © 2019 by the author(s). Distributed -

Class II MHC Cytoplasmic Domain-Mediated Signaling in B Cells a Tail of Two Signals
Human Immunology 80 (2019) 32–36 Contents lists available at ScienceDirect Human Immunology journal homepage: www.elsevier.com/locate/humimm Class II MHC cytoplasmic domain-mediated signaling in B cells: A tail of two T signals Jonathan A. Harton Department of Immunology & Microbial Disease, Albany Medical College, 47 New Scotland Avenue, MC-151, Albany, NY 12208, USA ARTICLE INFO ABSTRACT Keywords: In addition to their role in antigen presentation, class II MHC molecules also transmit signals to B lymphocytes. MHC class II Class II MHC-mediated signals initiate a range of events in B cells, including induction of cell surface proteins, HLA-D initiation of cell-cycle progression/proliferation, activation of or protection from apoptosis, and antigen-de- Signaling pendent plasma cell differentiation. Although various transmembrane signaling proteins associate with classII B cells MHC molecules, the class II MHC cytoplasmic domains are essential for signals leading to increased intracellular cAMP and activation of protein kinase C (PKC). Although truncation and mutagenesis studies have provided considerable information about the cytoplasmic domain sequences required, how class II MHC molecules elicit cAMP and PKC activation is not known. Further, appropriate T-dependent B cell responses require intact cAMP and PKC signaling, but the extent to which class II MHC signals are involved is also unknown. This review details our current knowledge of class II MHC cytoplasmic domain signaling in B cells with an emphasis on the likely importance of class II MHC signals for T-dependent antibody responses. 1. Introduction complex, is only found on CD4+ T cells which are dependent upon thymic MHC class II for their development. -

Cell Biology from an Immune Perspective in This Lecture We Will
Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Cell Biology from an Immune Perspective In this lecture we will very briefly review some aspects of cell biology which are required as background knowledge in order to understand how the immune system works. These will include: 1. A brief overview of protein trafficking 2. Signal transduction 3. The cell cycle Some of these issues will be treated in greater depth in later lectures. Protein Trafficking/The Secretory Pathway: From an immune perspective the secretory compartment and structures enclosed by vesicles are “seen” in different ways from proteins that reside in the cytosol or the nucleus. We will briefly review the secretory and endocytic pathways and discuss the biogenesis of membrane proteins. Some of the issues that will be discussed are summarized in Figures 1-3. Early endosomes Late endosomes Lysosomes Golgi Vesiculo-Tubular Compartment ER Figure 1. An overview of the secretory pathway Early endosomes Late endosomes Multivesicular and multilamellar bodies Golgi ER Proteasomes Figure 2. Protein degradation occurs mainly in lysosomes and proteasomes Proteins that enter the cell from the environment are primarily degraded in lysosomes. Most cytosolic and nuclear proteins are degraded in organelles called proteasomes. Intriguingly these two sites of degradation are each functionally linked to distinct antigen presentation pathways, different kinds of MHC molecules and the activation of different categories of T cells. Integral membrane proteins maybe inserted into the membrane in a number of ways, the two most common of these ways being considered in Figure 3. -

High-Allelic Variability in HLA-C Mrna Expression: Association with HLA-Extended Haplotypes
Genes and Immunity (2014) 15, 176–181 & 2014 Macmillan Publishers Limited All rights reserved 1466-4879/14 www.nature.com/gene ORIGINAL ARTICLE High-allelic variability in HLA-C mRNA expression: association with HLA-extended haplotypes F Bettens1,2, L Brunet1,2 and J-M Tiercy1 Human leukocyte antigen (HLA)-C is a clinically relevant transplantation antigen in unrelated hematopoietic stem cell and cord blood transplantation. Furthermore, HLA-C antigens, as ligands for killer immunoglobulin-like receptors expressed on natural killer cells, have a central role in HIV control. Several studies have reported significant correlations between HLA-C mRNA and cell surface expression with polymorphisms in the 50- and 30-regions of the HLA-C locus. We determined HLA-C mRNA in blood donors by using locus as well as allele-specific real-time–PCR and focused the analysis on HLA-extended haplotypes. High inter-individual variability of mRNA expression was disclosed. A lower inter-individual variability for C*07:01 but a higher variability for C*06:02, C*04:01 and C*03:04 alleles were detected. The previously reported associations between HLA-C cell surface expression and À 32 kb/ À 35 kb single nucleotide polymorphisms were not confirmed. Related and unrelated individuals sharing the same two A-B-C-DRB1 or B-C haplotypes show strikingly similar levels of HLA-C mRNA expression in each of the different haplotypic combinations tested. Altogether, our results suggest that HLA-C expression levels best correlate with the extended HLA haplotype rather than with the allotype or with polymorphisms in the 50-region of the HLA-C locus. -

MHC Class II Stabilization at the Surface of Human Dendritic Cells Is the Result of Maturation-Dependent MARCH I Down-Regulation
MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation Aude De Gassart*†‡, Voahirana Camosseto*†‡, Jacques Thibodeau§¶, Maurizio Ceppi*†‡, Nadia Catalan*†‡, Philippe Pierre*†‡ʈ, and Evelina Gatti*†‡ʈ *Centre d’Immunologie de Marseille–Luminy, Universite´ delaMe´ diterrane´e, Case 906, 13288 Marseille Cedex 9, France; †Institut National de la Sante´etde la Recherche Me´dicale, Unite´631, 13288 Marseille, France; ‡Centre National de la Recherche Scientifique, Unite´Mixte de Recherche 6102, 13288 Marseille, France; §De´partement de Microbiologie et Immunologie, Universite´de Montre´al, Montreal, QC, Canada H3T 1J4; and ¶Institut National de la Sante´et de la Recherche Me´dicale, Unite´743, Montreal, QC, Canada H3T 1J4 Edited by Ralph M. Steinman, The Rockefeller University, New York, NY, and approved January 9, 2008 (received for review September 19, 2007) In response to Toll-like receptor ligands, dendritic cells (DCs) dramatically when cells are exposed to LPS (12, 13). As determined dramatically enhance their antigen presentation capacity by sta- by confocal microscopy, iDCs accumulate HLA-DR molecules in bilizing at the cell-surface MHC II molecules. We demonstrate here LAMP1ϩ late endosomes and lysosomes. On activation by LPS, that, in human monocyte-derived DCs, the RING-CH ubiquitin E3 rapid relocalization of MHC II to the cell surface is observed (4–8 ligase, membrane-associated RING-CH I (MARCH I), promotes the h) [supporting information (SI) Fig. 7A]. Recently, this process has ubiquitination of the HLA-DR -chain. Thus, in nonactivated DCs, been shown to be associated with a loss of ubiquitination on the MARCH I induces the surface internalization of mature HLA-DR class II -chain in mouse bone marrow-derived DCs (bmDCs) (4, complexes, therefore reducing their stability and levels. -

Interaction Between CD8 and Major Histocompatibility Complex (MHC) Class I Mediated by Multiple Contact Surfaces That Include Th
Interaction Between CD8 and Major Histocompatibility Complex (MHC) Class I Mediated by Multiple Contact Surfaces that Include the oL2 and oL3 Domains of MHC Class I By Jiaren Sun,* DanielJ. Leahy,~ and Paula B. Kavathas*w From the *Department of Laboratory Medicine and Section of lmmunobiology, Yale University School of Medicine, New Haven, Connecticut 06520-8035; "Ji.Departmentof Biophysics and Biophysical Chemistry,Johns Hopkins University School of Medicine, Baltimore, Maryland 21205-2185; wDepartment of Genetics, Yale University School of Medicine, New Haven, Connecticut 06520-8035 Summary Downloaded from http://rupress.org/jem/article-pdf/182/5/1275/1107333/1275.pdf by guest on 03 October 2021 The cell surface glycoprotein CD8 functions as a coreceptor with the TCR on cytotoxic T lymphocytes. Mutational analysis of the binding site of CD8 for MHC class I predicted that distinct surfaces of CD8 would interact with both the a2 and a3 domains of class I. Using a cell-cell adhesion assay, we identified three residues Q115, D122, and E128 in the or2 domain of class I critical for interaction with CD8. The side chains of these residues point towards a cavity formed by the 0tl/e~2 platform, the or3 domain and [32-microglobulin ([32m) of class I. These residues were predicted to contact CD8 based on a bivalent model of interaction be- tween one CD8o~/oL homodimer and two MHC class I molecules. These results therefore pro- vide support for the model. HC class I molecules are highly polymorphic pro- critical residues in the 0~2 domain, two located underneath M teins that bind antigenic peptides and present them the peptide-binding floor and one on a nearby loop, all to T cells. -

Footprints of Antigen Processing Boost MHC Class II Natural Ligand
Barra et al. Genome Medicine (2018) 10:84 https://doi.org/10.1186/s13073-018-0594-6 RESEARCH Open Access Footprints of antigen processing boost MHC class II natural ligand predictions Carolina Barra1† , Bruno Alvarez1†, Sinu Paul2, Alessandro Sette2, Bjoern Peters2, Massimo Andreatta1, Søren Buus3 and Morten Nielsen1,4* Abstract Background: Major histocompatibility complex class II (MHC-II) molecules present peptide fragments to T cells for immune recognition. Current predictors for peptide to MHC-II binding are trained on binding affinity data, generated in vitro and therefore lacking information about antigen processing. Methods: We generate prediction models of peptide to MHC-II binding trained with naturally eluted ligands derived from mass spectrometry in addition to peptide binding affinity data sets. Results: We show that integrated prediction models incorporate identifiable rules of antigen processing. In fact, we observed detectable signals of protease cleavage at defined positions of the ligands. We also hypothesize a role of the length of the terminal ligand protrusions for trimming the peptide to the MHC presented ligand. Conclusions: The results of integrating binding affinity and eluted ligand data in a combined model demonstrate improved performance for the prediction of MHC-II ligands and T cell epitopes and foreshadow a new generation of improved peptide to MHC-II prediction tools accounting for the plurality of factors that determine natural presentation of antigens. Keywords: MHC-II, Binding predictions, Eluted ligands, T cell epitope, Neural networks, Antigen processing, Machine learning, Mass spectrometry Background MHC-II binding groove, unlike MHC class I, is open at Major histocompatibility complex class II (MHC-II) mole- both ends. -
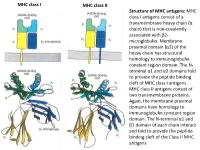
MHC Class I Antigens Consist of a Transmembrane Heavy Chain (Α Chain) That Is Non-Covalently Associated with Β2- Microglobulin
MHC class I MHC class II Structure of MHC antigens: MHC class I antigens consist of a transmembrane heavy chain (α chain) that is non-covalently associated with β2- microglobulin. Membrane proximal domain (α3) of the heavy chain has structural homology to immunoglobulin constant region domain. The N- terminal α1 and α2 domains fold to provide the peptide binding cleft of MHC class I antigens. MHC class II antigens consist of two transmembrane proteins. Again, the membrane proximal domains have homology to immunoglobulin constant region domain. The N-terminal α1 and β1 domain of each chain interact and fold to provide the peptide binding cleft of the Class II MHC antigens MHC class I MHC class II A top-down view of the peptide binding cleft of MHC antigens. The peptide binding cleft for MHC class I antigens is relatively narrower at the ends and accommodate 8- 10 amino acid residues long peptides. In contrast, due to non-covalent association between the N-terminal domains of the two chains of the class II antigens, the peptide binding cleft is open at the ends. This permits binding of larger peptides, typically 13-20 aa long. T cell receptor Interaction between T cell receptor and MHC-peptide complex: The T cell receptors binds to the peptide-MHC complex in a diagonal fashion. The CDR1 and CDR2 regions of the α and β chains of the T cell receptor bind to the α-helical walls of the peptide binding cleft. The CDR3 regions on the other hand bind to the peptide present in the cleft. -

HLA-DPB1 Gene Major Histocompatibility Complex, Class II, DP Beta 1
HLA-DPB1 gene major histocompatibility complex, class II, DP beta 1 Normal Function The HLA-DPB1 gene provides instructions for making a protein that plays a critical role in the immune system. The HLA-DPB1 gene is part of a family of genes called the human leukocyte antigen (HLA) complex. The HLA complex helps the immune system distinguish the body's own proteins from proteins made by foreign invaders such as viruses and bacteria. The HLA complex is the human version of the major histocompatibility complex (MHC), a gene family that occurs in many species. The HLA-DPB1 gene belongs to a group of MHC genes called MHC class II. MHC class II genes provide instructions for making proteins that are present on the surface of certain immune system cells. These proteins attach to protein fragments (peptides) outside the cell. MHC class II proteins display these peptides to the immune system. If the immune system recognizes the peptides as foreign (such as viral or bacterial peptides), it triggers a response to attack the invading viruses or bacteria. The protein produced from the HLA-DPB1 gene attaches (binds) to the protein produced from another MHC class II gene, HLA-DPA1. Together, they form a functional protein complex called an antigen-binding DPab heterodimer. This complex displays foreign peptides to the immune system to trigger the body's immune response. Each MHC class II gene has many possible variations, allowing the immune system to react to a wide range of foreign invaders. Researchers have identified hundreds of different versions (alleles) of the HLA-DPB1 gene, each of which is given a particular number (such as HLA-DPB1*03:01). -

HLA on Chromosome 6: the Story Gets Longer and Longer Leslie J
COMMENTARY HLA on Chromosome 6: The Story Gets Longer and Longer Leslie J. Raffel,1 Janelle A. Noble,2 and Jerome I. Rotter1 within the MHC has played a substantial role in allowing disease linkages and associations to be detected. The early ver the past three decades, substantial progress studies that reported associations with HLA-B8 and -B15 has been made in understanding the genetic used remarkably small numbers of cases and controls basis for diabetes. One of the important first relative to the hundreds to thousands considered neces- Osteps that allowed this progress to occur was sary in current studies (4–6). Had it not been for the realizing that diabetes is heterogeneous, and, therefore, strong LD, those initial studies would have been unlikely separation of clinically distinct forms of the disorder (i.e., to yield positive results. Yet, at the same time, this LD has type 1 vs. type 2 diabetes) improves the ability to detect also created challenges in accomplishing the next step, genetic associations. The key points that allowed type 1 that of identification of the specific genes that are respon- diabetes to be separated from type 2 diabetes included sible for disease susceptibility. The fact that researchers realization of the clinical differences (typically childhood have spent Ͼ30 years trying to elucidate all of the loci onset, thin, ketosis-prone versus adult onset, obese, non- responsible for type 1 diabetes susceptibility in the HLA ketosis prone); family and twin studies that demonstrated region underscores the complexity -

A Second Lineage of Mammalian Major Histocompatibility Complex Class I Genes SEIAMAK BAHRAM*, MAUREEN BRESNAHAN*, DANIEL E
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 6259-6263, July 1994 Immunology A second lineage of mammalian major histocompatibility complex class I genes SEIAMAK BAHRAM*, MAUREEN BRESNAHAN*, DANIEL E. GERAGHTYt, AND THOMAS SPIES* *Diision of Tumor Virology, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney Street, Boston, MA 02115; and tHuman Immunogenetics Program, Fred Hutchinson Cancer Research Center, 1124 Columbia Street, Seattle, WA 98104 Communicated by Sherman M. Weissman, January 3, 1994 ABSTRACT Major sctibit complex (MHC) polymorphic antigen-presenting molecules (2). The physio- class I genes tpically encode polymorphic peptide-binding logical roles of HLA-E and -F are uncertain (14, 15), but chains which are ubi ly expressed and mediate the HLA-G may have a specific function at the maternal-fetal recognition of intr rtigens by cytotoxic T cells. They interface (16). In addition, the MHC contains a number of constte diverse gene fme in different species and include class I pseudogenes and gene fragments (17); however, all of the numerous cd n acal genes in the mouse H-2 the human class I sequences share close relationships indi- coplex, of which some have been adapted to variously mod- cating their common origin from a typical class I gene. ified hmctions. We have identified a d nt family of five In this report, we describe a family of sequencest in the related sequenes in the human MHC whicb are distntly human MHC which are highly divergent from al of the homologous to dass I i. These MIC genes (MHC class I known MHC class I chains and have presumably been chain-related genes) evolved in parallel with the human class I derived early in the evolution of mammalian class I genes. -

Fcγ Receptors As Regulators of Immune Responses
REVIEWS Fcγ receptors as regulators of immune responses Falk Nimmerjahn* and Jeffrey V. Ravetch‡ Abstract | In addition to their role in binding antigen, antibodies can regulate immune responses through interacting with Fc receptors (FcRs). In recent years, significant progress has been made in understanding the mechanisms that regulate the activity of IgG antibodies in vivo. In this Review, we discuss recent studies addressing the multifaceted roles of FcRs for IgG (FcγRs) in the immune system and how this knowledge could be translated into novel therapeutic strategies to treat human autoimmune, infectious or malignant diseases. Regulatory T cells A productive immune response results from the effec- of antigenic peptides that are presented on the surface A T cell subset that is capable tive integration of positive and negative signals that of DCs to cytotoxic T cells, T helper cells, and regulatory of suppressing the activity have an impact on both innate and adaptive immune T cells. Thus, FcγRs are involved in regulating a multitude of other antigen-specific T cells cells. When positive signals dominate, cell activation of innate and adaptive immune responses, which makes including autoreactive T cells. Depletion of regulatory and pro-inflammatory responses ensue, resulting in the them attractive targets for the development of novel T cells results in the loss of elimination of pathogenic microorganisms and viruses. immunotherapeutic approaches (FIG. 1). peripheral tolerance and the In the absence of such productive stimulation, cell activa- In this Review, we summarize our current under- development of autoimmune tion is blocked and active anti-inflammatory responses standing of the role of different members of the FcγR disease.