Lichenoconium (Sphaeropsidales)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Og Fødevareudvalget 2016-17 MOF Alm.Del Endeligt Svar På Spørgsmål 776 Offentligt
Miljø- og Fødevareudvalget 2016-17 MOF Alm.del endeligt svar på spørgsmål 776 Offentligt Liste over ansøgere om landbrugsstøtte, der i kalenderåret 2016 fik udbetalt 1 million kr. eller mere. CVR nr Navn Postnr By Beløb 18387999 2 K Kristensen I/S 6971 Spjald 1.204.370,57 30859340 4g I/S v/H. P. & G. Garth-Gruner 4100 Ringsted 1.840.847,70 56986111 A/S Saltbækvig 2970 Hørsholm 1.495.301,75 34455589 AB-AGRO ApS 9370 Hals 1.688.949,45 21802247 Abildskovgård ApS 5672 Broby 1.795.485,74 30554175 Abildtrup Agro ApS 7560 Hjerm 1.195.261,45 28970838 Adamshøj Gods A/S 4100 Ringsted 1.340.454,91 65110768 Adolf Friedrich Bossen 6270 Tønder 1.406.503,11 74823513 Advokat Henrik Skaarup 9700 Brønderslev 2.404.609,69 29657823 Agrifos I/S 4912 Harpelunde 3.726.461,23 36073209 Agro Seeds ApS 7870 Roslev 1.605.902,94 65328313 Aksel Lund 6280 Højer 1.399.805,49 26118182 Akset A/S 7323 Give 1.018.414,87 44210851 Aktivitetscenter Vestervig-Agger 7770 Vestervig 1.194.484,00 26779642 Albæk I/S 6900 Skjern 1.237.617,84 19470989 Alex Ostersen 6900 Skjern 1.622.978,43 21044237 Alfred Ebbesen 6780 Skærbæk 1.087.666,28 21767247 Alfred Kloster 7490 Aulum 1.101.410,01 58565717 Allan Jensen 4791 Borre 1.215.043,67 35590439 Allan Møller Koch 6500 Vojens 2.098.744,28 36556498 Almende ApS 6270 Tønder 2.475.146,19 17804502 Anders Christensen 4640 Faxe 1.200.120,61 25675274 Anders Christiansen 7570 Vemb 1.106.339,88 13971692 Anders D. -

Euriskodata Rare Book Series
THE LIBRARY OF THE UNIVERSITY OF CALIFORNIA RIVERSIDE HI S T KY o F SCANDINAVIA. HISTOEY OF SCANDINAVIA. gxm tilt €mI% f iiius NORSEMEN AND YIKINGS TO THE PRESENT DAY. BY THE EEV. PAUL C. SINDOG, OF COPENHAGEN. professor of t^e Scanlimafaian fLanguagts anD iLifnaturr, IN THE UNIVERSITY OF THE CITY OF NEW-YORK. Nonforte ac temere humana negotia aguntur atque volvuntur.—Curtius. SECOND EDITION. NEW-YORK: PUDNEY & RUSSELL, PUBLISHERS. 1859. Entered aceordinfj to Act of Congress, in the year 1858, By the rev. PAUL C. SIN DING, In the Clerk's Office of the District Court of the United States, for the Southern Distriftt of New-York. TO JAMES LENOX, ESQ., OF THE CUT OF NEW-TOBK, ^ht "^nu of "^ttttxs, THE CHIIISTIAN- GENTLEMAN, AND THE STRANGER'S FRIEND, THIS VOLUME IS RESPECTFULLY INSCRIBED, BY THE AUTHOR PREFACE. Although soon after my arrival in the city of New-York, about two years ago, learning by experience, what already long had been known to me, the great attention the enlightened popu- lation of the United States pay to science and the arts, and that they admit that unquestion- able truth, that the very best blessings are the intellectual, I was, however, soon . aware, that Scandinavian affairs were too little known in this country. Induced by that ardent patriotism peculiar to the Norsemen, I immediately re- solved, as far as it lay in my power, to throw some light upon this, here, almost terra incog- nita, and compose a brief History of Scandinavia, which once was the arbiter of the European sycjtem, and by which America, in reality, had been discovered as much as upwards of five Vlll PREFACE centuries before Columbus reached St. -
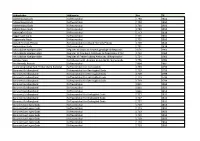
Skifteprotokoller, Sjælland
Skifteprotokoller, Sjælland Arkivskaber Arkivserie Fra Til Adelersborg Gods Skifteprotokol 1790 1850 Adelersborg Gods Skifteprotokol 1790 1850 Adelersborg Gods Skifteprotokol 1790 1850 Adelersborg Gods Skifteprotokol 1790 1850 Agersøgård Gods Skifteprotokol 1773 1818 Aggersvold Gods Skifteprotokol 1721 1850 Aggersvold Gods Skifteprotokol 1721 1850 Alsted Herreds Provsti Skifteprotokol for Alsted Herreds Provsti 1759 1814 Antvorskov Gods Skifteprotokol 1791 1818 Arkivskabte Hjælpemidler Register til Smørum Herreds gejstlige skifteprotok 1676 1960 Arkivskabte Hjælpemidler Register til Kronborg Amtstues skifteprotokol 1712 1712 1960 Arkivskabte Hjælpemidler Register til Frederiksborg Amtstues skifteprotokol 1724 1960 Arninge Sogn Skiftebreve vedr. Arninge præstekalds mensalgods 1776 1781 Ars Herreds Provsti Skifteprotokol 1804 1807 Asminderød-Grønholt-Fredensborg Pastorat Skifteprotokol for mensalgods 1799 1799 Baroniet Guldborgland Skifteprotokol for Berritsgård Gods 1719 1799 Baroniet Guldborgland Skifteprotokol for Berritsgård Gods 1719 1799 Baroniet Guldborgland Skifteprotokol for Berritsgård Gods 1719 1799 Baroniet Guldborgland Skifteprotokol for Berritsgård Gods 1719 1799 Baroniet Guldborgland Skifteprotokol 1811 1850 Baroniet Guldborgland Skifteprotokol 1811 1850 Baroniet Guldborgland Skifteprotokol 1811 1850 Baroniet Guldborgland Skifteprotokol for Orebygård Gods 1727 1811 Baroniet Guldborgland Skifteprotokol for Orebygård Gods 1727 1811 Baroniet Guldborgland Skifteprotokol for Orebygård Gods 1727 1811 Baroniet Juellinges Gods Skifteprotokol -

Fortid Og Nutid 1960. 21.Bd. 1.Hf
Dette værk er downloadet fra Slægtsforskernes Bibliotek SLÆGTSFORSKERNES BIBLIOTEK Slægtsforskernes Bibliotek drives af foreningen Danske Slægtsforskere. Det er et special-bibliotek med værker, der er en del af vores fælles kulturarv, blandt andet omfattende slægts-, lokal- og personalhistorie. Slægtsforskernes Bibliotek: http://bibliotek.dis-danmark.dk Foreningen Danske Slægtsforskere: www.slaegtogdata.dk Bemærk, at biblioteket indeholder værker både med og uden ophavsret. Når det drejer sig om ældre værker, hvor ophavsretten er udløbet, kan du frit downloade og anvende PDF-filen. Drejer det sig om værker, som er omfattet af ophavsret, skal du være opmærksom på, at PDF- filen kun er til rent personlig brug. TIDSSKRIFT FOR KULTURHISTORIE OG LOKALHISTORIE UDGIVET AF DANSK HISTORISK FÆLLESFORENING FORTID ogNUTID BIND XXI . HEFTE 1 . SIDE 1 — 112 . KØBENHAVN 1960 Statens Arkiv for Historiske Film og Stemmer Af D. Yde-Andersen Statens Arkiv går tilbage til 1911. I dette år blev det norske dagblad Ver dens Gang og den danske filmkonge Ole Olsen dybt uenige. Verdens Gang henvendte sig til Politiken om kollegial støtte, og Henrik Cavling over drog det til sin unge medarbejder Anker Kirkeby at udføre eksekutionen. Anker Kirkeby var en journalist af format. Han så, at der kunne komme noget nyttigt ud af striden. Der var tid efter anden fremkommet med delelser om, at man i udlandet ville oprette arkiver indeholdende film, der skildrede store begivenheder eller kendte personer, til brug for histo rieforskningen. Nu mente Anker Kirkeby, der var mulighed for at få op rettet et sådant arkiv herhjemme. Han gik til Ole Olsen og tilbød at skaffe ham fred med Verdens Gang, mod at Ole Olsens selskab, Nordisk Films Kompagni, optog en række »kinematografiske Portrætter af kendte Per sonligheder, som det maa være af historisk Interesse at faa overleveret Efterverdenen«. -

To Fynske Herregårde Under Louis Seize-Tiden: Krengerup Og Einsidelsborg
Fynske Årbøger 1975 To fynske herregårde under Louis Seize-tiden: Krengerup og Einsidelsborg Af Claus M. Smidt Ved skæbnens gunst er vor Louis Seize-arkitektur i provinsen for trinsvis blevet præget af elever af arkitekten N.-H. Jardin. En 1 særlig rig del af denne er - som godtgjort af Chr. Elling ) - den fynske. Et af de mest imponerende herregårdsanlæg fra denne tid er Krengerup på Fyn. Over hoveddøren står indskriften "Af Fried rich Siegfried Baron af Rantzau Ridder, kammerherre og oberst og hans frue Sophie Magdalena født Baronesse Juell-Wind er dette hus opbygget 1772". Elling har påvist, at indskriften mere er et fingerpeg end en eksakt oplysning, der står til troende. Nævnte år var Rantzau hverken ridder, kammerherre, oberst eller ægtemand. Først 1776 opfyldte han dette, hvorfor den indi rekte tale er: begyndt 1772 og fuldendt omkring 1776 eller der efter. Om bygningens arkitekt ved samtiden intet at sige. Et kunst 2 nerleksikon fra 1829 angiver dog denne som Hans Næss. ) Næss var født på Fyn 1723 som søn af bønder fra Assens-eg nen.3) Som ung kom han på godskontoret på Brahesborg hos grev Chr. Rantzau. Derpå gik vejen til amtsstuen i Assens. I 1758 foretog han det store spring til København. Rantzau havde anbefa let ham til grev Otto Thott, der skaffede ham en volontørplads i Rentekammeret Samtidig hermed begyndte han på Kunstakade miet. Efter at have gennemgået arkitekturskolen og have fået alle dennes medaljer, inclusive den store guldmedalje, blev Næss 1765 informator i bygningskunsten og forlod derfor Rentekam- Fynske Årbøger 1975 To fynske hen·egårde under Louis Seize-tiden 73 meret. -

44259 - Materie 16/08/04 7:27 Side I
A Windfall for the Magnates. The Development of Woodland Ownership in Denmark c. 1150-1830 Fritzbøger, Bo Publication date: 2004 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Fritzbøger, B. (2004). A Windfall for the Magnates. The Development of Woodland Ownership in Denmark c. 1150-1830. Syddansk Universitetsforlag. Download date: 29. Sep. 2021 44259 - Materie 16/08/04 7:27 Side i “A Windfall for the magnates” 44259 - Materie 16/08/04 7:27 Side ii Denne afhandling er af Det Humanistiske Fakultet ved Københavns Universitet antaget til offentligt at forsvares for den filosofiske doktorgrad. København, den 16. september 2003 John Kuhlmann Madsen Dekan Forsvaret finder sted fredag den 29. oktober 2004 i auditorium 23-0-50, Njalsgade 126, bygning 23, kl. 13.00 44259 - Materie 16/08/04 7:27 Side iii “A Windfall for the magnates” The Development of Woodland Ownership in Denmark c. 1150-1830 by Bo Fritzbøger University Press of Southern Denmark 2004 44259 - Materie 16/08/04 7:27 Side iv © The author and University Press of Southern Denmark 2004 University of Southern Denmark Studies in History and Social Sciences vol. 282 Printed by Special-Trykkeriet Viborg a-s ISBN 87-7838-936-4 Cover design: Cover illustration: Published with support from: Forskningsstyrelsen, Danish Research Agency The University of Copenhagen University Press of Southern Denmark Campusvej 55 DK-5230 Odense M Phone: +45 6615 7999 Fax: +45 6615 8126 [email protected] www.universitypress.dk Distribution in the United States and Canada: International Specialized Book Services 5804 NE Hassalo Street Portland, OR 97213-3644 USA Phone: +1-800-944-6190 www.isbs.com 44259 - Materie 16/08/04 7:27 Side v Contents Preface . -

Danmarks Nationalbank Report and Accounts
Danmarks Nationalbank 2005 Danmarks Report andAccounts Nationalbank Report and Accounts 05 Danmarks Nationalbank Havnegade 5 DK-1093 Copenhagen K D A N M A R K S Telephone +45 33 63 63 63 Fax +45 33 63 71 03 N A T I O N A L www.nationalbanken.dk E-mail: [email protected] B A N K 2 0 0 5 aab_DK_06.indd 1 10-02-2006 13:59:59 Report and Accounts 2005 REPORT AND ACCOUNTS 2005 At the meeting of the Board of Directors held on 20 March 2006 the Board of Governors reported on the activities of Danmarks Nationalbank. The report was noted. Danmarks Nationalbank's accounts for 2005 were submitted by the Board of Governors for adoption on the recommendation of the Committee of Directors. The Board of Directors and the Royal Bank Commissioner accepted the recommendation. This Report is based on information available up to 2 March 2006. The small picture on the front cover is a section of the fairy tale coin "The Little Mermaid", which is the second coin in a series of five with fairy tales as their common theme. The motif was designed by the sculptor Tina Maria Nielsen. Text may be copied from this publication provided that Danmarks Nationalbank is specific- ally stated as the source. Changes to or misrepresentation of the content are not permit- ted. The Report and Accounts is available on Danmarks Nationalbank's website: www.nationalbanken.dk under Publications and can be ordered by filling in the form on Danmarks Nationalbank's website. The Report and Accounts 2005 is also available on request from: Danmarks Nationalbank Information Desk Havnegade 5 DK-1093 Copenhagen K Telephone: (+45) 33 63 70 00 (direct) or (+45) 33 63 63 63 Office hours: Monday-Friday 9.00 am-4.00 pm. -

Odense Adelige Jomfrukloster Havde Found Their Way to the Archives
198 Th e Arcade Room Who lived in the so-called Arcade Room, 206, is not redecorated before moving in. Th e conservators known before the end of the 1780’s, but the room’s estimate that the painted wooden walls were lined appearance has changed completely several times with canvas and wallpaper between 1780 and 1800, – none of the other rooms on the fi rst fl oor have so it may have been Miss de Leth who had the room had such diff erent styles within the course of a few decorated – perhaps aft er 45 years at the dilapidated decades. Sanderumgaard she needed to see something fresh In 1785, the 38-year old Johanne de Leth of and modern. Sanderumgaard Manor became a conventual. She Johanne de Leth lived here until 1808 when she came from a family of 14 siblings, and of the eight became Prioress and moved down to the ground daughters, one was dead and three had long been fl oor. She died at the age of 77 in 1825 and was buried married. Her father had died a few years before, and in St. Knud’s Church. Th e church register states that she now lived at the manor together with her mother the bells were rung – this was not otherwise done and two unmarried sisters in their thirties. Th is for Prioresses or ladies, no doubt because of the was not the present Sanderumgaard, whose main bell-ringer’s fee. building dates from the 1870’s. Johan von Bülow, One of Miss de Leth’s unmarried sisters entered who bought the manor aft er the mother’s death in Vemmetoft e Secular Convent for Noblewomen, but, 1792, described it in his diary as a modest and very even so, lived for a while with a sister and brother- dilapidated house in one storey, with small leaded in-law in Kerteminde. -

Getting to Denmark’: the Role of Elites for Development
Working Papers in Economic History February 2018 WP 18-03 Getting to Denmark’: the Role of Elites for Development Peter Sandholt Jensen Markus Lampe Paul Sharp Christian Volmar Skovsgaard Abstract We explore the role of elites for development and in particular for the spread of cooperative creameries in Denmark in the 1880s, which was a major factor behind that country’s rapid economic catch-up. We demonstrate empirically that the location of early proto-modern dairies, so-called hollænderier, introduced onto traditional landed estates as part of the Holstein System of agriculture by landowning elites from the Duchies of Schleswig and Holstein in the eighteenth century, can explain the location of cooperative creameries in 1890, more than a century later, after controlling for other relevant determinants. We interpret this as evidence that areas close to estates which adopted the Holstein System witnessed a gradual spread of modern ideas from the estates to the peasantry. Moreover, we identify a causal relationship by utilizing the nature of the spread of the Holstein System around Denmark, and the distance to the first estate to introduce it, Sofiendal. These results are supported by evidence from a wealth of contemporary sources and are robust to a variety of alternative specifications.. Keywords: Institutions, technology, knowledge spillovers, landowning elites, cooperatives, Denmark JEL Classification: N53, O13, Q13 Markus Lampe (corresponding author): Institute for Economic and Social History, Vienna University of Economics and Business (Welthandelsplatz 1, Bg D4, 1020 Vienna, Austria) and Instituto Figuerola. E-mail: [email protected] https://www.wu.ac.at/geschichte/institut/personal/lampe-markus/ Publisher: Carlos III University of Madrid. -
Oversigt Over Sjællandske Godsarkiver, Online Marts 2018
Oversigt over sjællandske godsarkiver, online marts 2018 Sjællandske godsarkiver Arkivalier Datering fra Datering til Aggersvold Gods Skiftedokumenter 1721 1758 Aggersvold Gods Skiftedokumenter 1758 1809 Aggersvold Gods Skiftedokumenter 1809 1843 Aggersvold Gods Skiftedokumenter 1843 1850 Bregentved Gods Skiftedokumenter for Godset i Sorø Amt 1790 1834 Bregentved Gods Skiftedokumenter for Godset i Sorø Amt 1834 1851 Valbygård Gods Jordebog 1841 Valbygård Gods Jordebog 1805 1841 Hardenberg-Reventlow Gods Mikrofilm fra Brandenburgisches Landeshauptarchiv 1750 1800 Sorø Akademis Gods Behandlingsprotokol 1790 1827 Sorø Akademis Gods Behandlingsprotokol 1827 1833 Sorø Akademis Gods Behandlingsprotokol 1832 1846 Sorø Akademis Gods Behandlingsprotokol 1846 1850 Sorø Akademis Gods Behandlingsprotokol 1838 1850 Krenkerup Gods Fæsteprotokol for Nielstrup Gods 1679 1824 Krenkerup Gods Fæsteprotokol for grevskabet Hardenberg-Reventlows 1826 1854 Krenkerup Gods Fæsteprotokol for grevskabet Hardenberg-Reventlows 1854 1924 Krenkerup Gods Fæsteprotokol for grevskabet Hardenberg-Reventlows 1830 1901 Krenkerup Gods Fæsteprotokol for Wintersborg og Sæbyholm Godser 1761 1830 Vibygård Gods Fæsteprotokol uden registratur 1719 1827 Giesegård Gods Fæsteprotokol 1720 1816 Giesegård Gods Fæsteprotokol 1816 1859 Bistrup Gods Fæsteprotokol 1694 1705 Bistrup Gods Fæsteprotokol 1728 1761 Bistrup Gods Fæsteprotokol 1841 1918 Vartov Hospitals Gods Fæsteprotokol 1723 1804 Ågård og Helsingegårds Godser Fæstebreve og kvittering for betalt landgilde 1756 1776 Åstrup Gods -

Stamps (Sellos
718 DENMARK printed on the back. Value for least costly The arabesques in the corners have a main 1884-88 DENMARK reprint of No. 2, $8.50. stem and a branch. When the frame is in nor- mal position, in the upper left corner the Larger Corner Numerals den-mark branch leaves the main stem half way between 38 A7 5o green 11.00 1.40 two little leaflets. In the lower right corner the a. Imperf. branch starts at the foot of the second leaflet. 39 A7 10o carmine (’85) 11.00 1.40 a. Small numerals in corners LOCATION — Northern part of a penin- When the frame is inverted the corner designs (’88) 400.00 525.00 sula which separates the North and are, of course, transposed. b. Imperf., single 140.00 c. Pair, Nos. 39, 39a 475.00 775.00 Baltic Seas, and includes the sur- 1 1870-71 Wmk. 112 Perf. 14x13 /2 40 A7 20o blue 17.50 1.75 rounding islands Paper Varying from Thin to Thick a. Pair, Nos. 37, 40 300.00 675.00 GOVT. — Kingdom b. Imperf. Dotting in Wavy Lines 16 A6 2s gray & ultra Nos. 38-40 (3) 39.50 4.55 AREA — 16,631 sq. mi. Spandrels in Spandrels (’71) 45.00 22.50 Set, never hinged 110.00 POP. — 5,294,860 (1/1/1999) A3 A4 a. 2s gray & blue 45.00 22.50 17 A6 3s gray & brt lil Stamps with large corner numerals have CAPITAL — Copenhagen (’71) 72.50 85.00 white line around crown and lower oval touches frame. -

University of Copenhagen
Egens sundhedsproblemer på grundvandsnære jorde Callesen, Ingeborg; Jørgensen, Bruno Bilde; Fischer, Lene; Larsen, Hanne Marie Ellegård; Ravn, Hans Peter; Susgaard Filsø, Stinna; Bjerager, Per Eduard Robert; Thomsen, Iben Margrete Publication date: 2017 Document version Også kaldet Forlagets PDF Citation for published version (APA): Callesen, I., Jørgensen, B. B., Fischer, L., Larsen, H. M. E., Ravn, H. P., Susgaard Filsø, S., ... Thomsen, I. M. (2017). Egens sundhedsproblemer på grundvandsnære jorde. Frederiksberg. IGN Rapport Download date: 08. Apr. 2020 københavns universitet institut for geovidenskab og naturforvalting Egens sundhedsproblemer på grundvandsnære jorde Ingeborg Callesen, Bruno Bilde Jørgensen, Lene Fischer, Hanne Marie Larsen, Hans Peter Ravn, Stinna Susgaard Filsø, Per Bjerager og Iben Margrete Thomsen IGN Rapport September 2017 Titel Egens sundhedsproblemer på grundvandsnære jorde Forfattere Ingeborg Callesen, Bruno Bilde Jørgensen, Lene Fischer, Hanne Marie Larsen, Hans Peter Ravn, Stinna Susgaard Filsø, Per Bjerager, Iben Margrete Thomsen Bedes citeret Ingeborg Callesen, Bruno Bilde Jørgensen, Lene Fischer, Hanne Marie Larsen, Hans Peter Ravn, Stinna Susgaard Filsø, Per Bjerager og Iben Margrete Thomsen (2017): Egens sundhedsproblemer på grund- vandsnære jorde. IGN Rapport, september 2017, Institut for Geoviden- skab og Naturforvaltning, Frederiksberg. 90 s. ill. Udgiver Institut for Geovidenskab og Naturforvaltning Københavns Universitet Rolighedsvej 23 1958 Frederiksberg C [email protected] www.ign.ku.dk Ansvarshavende