(Diplopoda: Callipodida: Schizopetalidae) from Bulgaria, with a Review of the A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Millipedes (Diplopoda) from Caves of Portugal
A.S.P.S. Reboleira and H. Enghoff – Millipedes (Diplopoda) from caves of Portugal. Journal of Cave and Karst Studies, v. 76, no. 1, p. 20–25. DOI: 10.4311/2013LSC0113 MILLIPEDES (DIPLOPODA) FROM CAVES OF PORTUGAL ANA SOFIA P.S. REBOLEIRA1 AND HENRIK ENGHOFF2 Abstract: Millipedes play an important role in the decomposition of organic matter in the subterranean environment. Despite the existence of several cave-adapted species of millipedes in adjacent geographic areas, their study has been largely ignored in Portugal. Over the last decade, intense fieldwork in caves of the mainland and the island of Madeira has provided new data about the distribution and diversity of millipedes. A review of millipedes from caves of Portugal is presented, listing fourteen species belonging to eight families, among which six species are considered troglobionts. The distribution of millipedes in caves of Portugal is discussed and compared with the troglobiont biodiversity in the overall Iberian Peninsula and the Macaronesian archipelagos. INTRODUCTION All specimens from mainland Portugal were collected by A.S.P.S. Reboleira, while collectors of Madeiran speci- Millipedes play an important role in the decomposition mens are identified in the text. Material is deposited in the of organic matter, and several species around the world following collections: Zoological Museum of University of have adapted to subterranean life, being found from cave Copenhagen, Department of Animal Biology, University of entrances to almost 2000 meters depth (Culver and Shear, La Laguna, Spain and in the collection of Sofia Reboleira, 2012; Golovatch and Kime, 2009; Sendra and Reboleira, Portugal. 2012). Although the millipede faunas of many European Species were classified according to their degree of countries are relatively well studied, this is not true of dependence on the subterranean environment, following Portugal. -

Kunst- Und Kulturbericht 2020 Kunst- Und Kulturbericht 2020
Kunst Kultur Bericht Kunst- und Kulturbericht 2020 Kunst- und Kulturbericht 2020 Wien 2021 Liebe Leserinnen und Leser, als wir im Juli des vergangenen Jahres den Kunst- und Kulturbericht 2019 veröffent- lichten, hatten wir gehofft, dass die optimistischen Prognosen über den Verlauf und die Eindämmung der Pandemie eintreten werden und wir Anfang des Jahres 2021 unser gesellschaftliches und kulturelles Leben langsam wieder aufnehmen können. Es kam anders. Das Virus und seine Mutanten haben es nicht erlaubt, dass wir uns ohne er- hebliche Gefahr für unsere Gesundheit in größeren Gruppen treffen oder als Publikum versammeln. Diese unsichtbaren Gegner haben es unmöglich gemacht, in Theatern, Kinos, Konzerthäusern, Galerien und Museen zusammenzukommen, um das zu tun, was wir alle lieben: gemeinsam Kunst zu erleben und zu genießen. Unser wichtigstes Ziel war es daher, alles zu unternehmen, damit wir am Ende der Pan- demie dort fortsetzen können, wo wir plötzlich und unerwartet aus unserem kulturellen Leben gerissen wurden: All jene, die in unserem Land Kunst machen, die sich dazu entschlossen haben, ihr Leben und ihre ganze Kraft dem künstlerischen Schaffen zu widmen, sollten bestmöglich durch diese schwierigen Zeiten kommen. All das, was in den vergangenen Jahren und Jahrzehnten an kultureller Infrastruktur entwickelt, aufgebaut und eingerichtet worden ist, sollte diese schwierigen Monate unbeschadet überstehen. Rasch, effizient und gezielt zu unterstützen, das war unsere politische Leitlinie, nach der wir die gesetzlichen Voraussetzungen geschaffen und die administrativen Prozesse aufgesetzt haben – und wir sind stolz darauf, dass uns vieles gelungen ist. So flossen im Jahr 2020 aus dem Covid-19-Krisenbewältigungsfonds der Bundesregierung zusätzliche Budgetmittel in dreistelliger Millionenhöhe direkt an Künstlerinnen und Künstler, an die Bundesmuseen, die Nationalbibliothek und den Bundestheater-Konzern sowie an Non-Profit-Organisationen im Bereich Kunst, Kultur und Denkmalpflege. -

Supra-Familial Taxon Names of the Diplopoda Table 4A
Milli-PEET, Taxonomy Table 4 Page - 1 - Table 4: Supra-familial taxon names of the Diplopoda Table 4a: List of current supra-familial taxon names in alphabetical order, with their old invalid counterpart and included orders. [Brackets] indicate that the taxon group circumscribed by the old taxon group name is not recognized in Shelley's 2003 classification. Current Name Old Taxon Name Order Brannerioidea in part Trachyzona Verhoeff, 1913 Chordeumatida Callipodida Lysiopetalida Chamberlin, 1943 Callipodida [Cambaloidea+Spirobolida+ Chorizognatha Verhoeff, 1910 Cambaloidea+Spirobolida+ Spirostreptida] Spirostreptida Chelodesmidea Leptodesmidi Brölemann, 1916 Polydesmida Chelodesmidea Sphaeriodesmidea Jeekel, 1971 Polydesmida Chordeumatida Ascospermophora Verhoeff, 1900 Chordeumatida Chordeumatida Craspedosomatida Jeekel, 1971 Chordeumatida Chordeumatidea Craspedsomatoidea Cook, 1895 Chordeumatida Chordeumatoidea Megasacophora Verhoeff, 1929 Chordeumatida Craspedosomatoidea Cheiritophora Verhoeff, 1929 Chordeumatida Diplomaragnoidea Ancestreumatoidea Golovatch, 1977 Chordeumatida Glomerida Plesiocerata Verhoeff, 1910 Glomerida Hasseoidea Orobainosomidi Brolemann, 1935 Chordeumatida Hasseoidea Protopoda Verhoeff, 1929 Chordeumatida Helminthomorpha Proterandria Verhoeff, 1894 all helminthomorph orders Heterochordeumatoidea Oedomopoda Verhoeff, 1929 Chordeumatida Julida Symphyognatha Verhoeff, 1910 Julida Julida Zygocheta Cook, 1895 Julida [Julida+Spirostreptida] Diplocheta Cook, 1895 Julida+Spirostreptida [Julida in part[ Arthrophora Verhoeff, -

A New Genus of the Millipede Tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean Region
European Journal of Taxonomy 70: 1-12 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2013.70 www.europeanjournaloftaxonomy.eu 2013 · Lazányi E. & Vagalinski B. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:5E23F454-2A68-42F6-86FB-7D9952B2FE7B A new genus of the millipede tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean region Eszter LAZÁNYI1,4 & Boyan VAGALINSKI2,3,5 1 Corresponding author: Department of Zoology, Hungarian Natural History Museum, Baross u. 13, H-1088 Budapest, Hungary. E-mail: [email protected] 2 Faculty of Biology, Sofia University, 8 Dragan Tsankov Blvd., 1164 Sofia, Bulgaria. E-mail: [email protected] 3 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Yurii Gagarin Street, 1113, Sofia, Bulgaria. 4 urn:lsid:zoobank.org:author:02DB48F1-624C-4435-AF85-FA87168CD85A 5 urn:lsid:zoobank.org:author:973B8725-039E-4F29-8D73-96A7F52CF934 Abstract. A new genus of the julid tribe Brachyiulini, Enghophyllum gen. nov., is described, comprising two species from Greece. The type-species, E. naxium (Verhoeff, 1901) comb. nov. (ex Megaphyllum Verhoeff, 1894), appears to be rather widespread in the Aegean: it is known from Antiparos Island and Naxos Island (the type locality), both in the Cyclades, as well as East Mavri Islet, Dodecanese Archipelago (new record). The vulva of E. naxium is described for the first time. In addition,E. sifnium gen. et sp. nov. is described based on a single adult male from Sifnos Island, Cyclades. The new genus is distinct from other genera of the Brachyiulini mainly by its peculiar gonopod structure, apparently disjunct and at least mostly apomorphous: (1) promeres broad, shield-like, in situ protruding mostly posteriad, completely covering the opisthomeres and gonopodal sinus; (2) transverse muscles and coxal apodemes of promere fully reduced; (3) opisthomere with three differentiated processes, i.e., lateral, basal posterior and apical posterior; (4) solenomere rather simple, tubular. -

MYRIAPODS 767 Volume 2 (M-Z), Pp
In: R. Singer, (ed.), 1999. Encyclopedia of Paleontology, MYRIAPODS 767 volume 2 (M-Z), pp. 767-775. Fitzroy Dearborn, London. MYRIAPODS JVlyriapods are many-legged, terrestrial arthropods whose bodies groups, the Trilobita, Chelicerata, Crustacea, and the Uniramia, the are divided into two major parts, a head and a trunk. The head last consisting of the Myriapoda, Hexapoda, and Onychophora (vel- bears a single pair of antennae, highly differentiated mandibles (or vet worms). However, subsequent structural and molecular evidence jaws), and at least one pair of maxillary mouthparts; the trunk indicates that there are several characters uniting major arthropod region consists of similar "metameres," each of which is a func- taxa. Moreover, paleobiologic, embryologie, and other evidence tional segment that bears one or two pairs of appendages. Gas demonstrates that myriapods and hexapods are fiindamentally exchange is accomplished by tracheae•a branching network of polyramous, having two major articulating appendages per embry- specialized tubules•although small forms respire through the ological body segment, like other arthropods. body wall. Malpighian organs are used for excretion, and eyes con- A fourth proposal (Figure ID) suggests that myriapods are sist of clusters of simple, unintegrated, light-sensitive elements an ancient, basal arthropod lineage, and that the Hexapoda that are termed ommatidia. These major features collectively char- emerged as an independent, relatively recent clade from a rather acterize the five major myriapod clades: Diplopoda (millipeds), terminal crustacean lineage, perhaps the Malacostraca, which con- Chilopoda (centipeds), Pauropoda (pauropods), Symphyla (sym- tains lobsters and crabs (Ballard et al. 1992). Because few crusta- phylans), and Arthropleurida (arthropleurids). Other features cean taxa were examined in this analysis, and due to the Cambrian indicate differences among these clades. -

Anamorphic Development of Apfelbeckia Insculpta (L. Koch, 1867) (Diplopoda: Callipodida: Schizopetalidae)
Arch Biol Sci. 2016;68(2):445-450 DOI:10.2298/ABS150802128I ANAMORPHIC DEVELOPMENT OF APFELBECKIA INSCULPTA (L. KOCH, 1867) (DIPLOPODA: CaLLIPODIDA: SCHIZOPETALIDAE) Bojan S. Ilić*, Vladimir T. Tomić, Luka R. Lučić and Bojan M. Mitić University of Belgrade – Faculty of Biology, Institute of Zoology, Studentski Trg 16, 11000 Belgrade, Serbia *Corresponding author: [email protected] Received: August 2, 2015; Revised: September 3, 2015; Accepted: September 16, 2015; Published online: November 3; 2015 Abstract: An overview of the anamorphic development of Apfelbeckia insculpta is provided. As in other myriapods and arthropods, post-embryonic period of the life cycle includes different stages that are separated by molts. Based on an earlier description of post-embryogenesis of A. insculpta and on our data, we describe ten stadia that occur after juveniles of our focal species hatch from the egg. Each molt is accompanied by the addition of podous and apodous pleurotergites, leg-pairs and ocelli. Thus, the numbers of these structures can be used as reliable criteria for the separation of post-embryonic sta- dia in A. insculpta. Adulthood is reached through teloanamorphosis, i.e., with the ninth and last molt individuals become sexually mature and achieve adulthood. Sexes can be distinguished from stadium VIII onward. Key words: Apfelbeckia insculpta; post-embryonic development; teloanamorphosis INTRODUCTION modes of anamorphosis are recognized in Diplopoda: euanamorphosis, hemianamorphosis, and teloan- Most millipedes can be described as multi-segmented amorphosis [4-6]. In euanamorphosis (recognized in and multi-legged animals. However, newly hatched Julida and Colobognatha), animals molt throughout millipedes usually have a significantly lower number life and every molt is accompanied by the addition of segments and legs than adults and the final, or adult of new segments. -

Full Issue, Vol. 57 No. 3
Great Basin Naturalist Volume 57 Number 3 Article 15 7-31-1997 Full Issue, Vol. 57 No. 3 Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation (1997) "Full Issue, Vol. 57 No. 3," Great Basin Naturalist: Vol. 57 : No. 3 , Article 15. Available at: https://scholarsarchive.byu.edu/gbn/vol57/iss3/15 This Full Issue is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. T H E GREAT BASBASINI1 naturalistnaturalist ale A VOLUME 57 ngN 3 JULY 1997 BRIGHAM YOUNG university GREAT BASIN naturalist editor assistant editor RICHARD W BAUMANN NATHAN M SMITH 290 MLBM 190 MLBM PO box 20200 PO box 26879 brigham youhgyoung university brigham young university provo UT 84602020084602 0200 provo UT 84602687984602 6879 8013785053801 378 5053 8017378801378668880173786688801 378 6688 FAX 8013783733801 378 3733 emailE mail nmshbllibyuedunmshbll1byuedu associate editors J R CALLAHAN PAUL C MARSH museum of southwestern biology university of tentercentergenter for environmental studies arizona new mexico albuquerque NM state university tempe AZ 85287 mailing address box 3140 hemet CA 92546 STANLEY D SMITH BRUCE D ESHELMAN department of biology department of Biologicbiologicalajlainaln sciences university of university of nevada las vegas wisconsin whitewawhitewaterwhitewayterten -

The Biological Resources of Illinois Caves and Other
I LLINOI S UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN PRODUCTION NOTE University of Illinois at Urbana-Champaign Library Large-scale Digitization Project, 2007. EioD THE BIOLOGICAL RESOURCES OF ILLINOIS CAVES AND OTHER SUBTERRANEAN ENVIRONMENTS Determination of the Diversity, Distribution, and Status of the Subterranean Faunas of Illinois Caves and How These Faunas are Related to Groundwater Quality Donald W. Webb, Steven J. Taylor, and Jean K. Krejca Center for Biodiversity Illinois Natural History Survey 607 East Peabody Drive Champaign, Illinois 61820 (217) 333-6846 TECHNICAL REPORT 1993 (8) ILLINOIS NATURAL HISTORY SURVEY CENTER FOR BIODIVERSITY Prepared for: The Environmental Protection Trust Fund Commission and Illinois Department of Energy and Natural Resources Office of Research and Planning 325 W. Adams, Room 300 Springfield, IL 62704-1892 Jim Edgar, Governor John Moore, Director State of Illinois Illinois Department of Energy and Natural Resources ACKNOWLEDGEMENTS Funding for this project was provided through Grant EPTF23 from the Environmental Protection Trust Fund Commission, administered by the Department of Energy and Natural Resources (ENR). Our thanks to Doug Wagner and Harry Hendrickson (ENR) for their assistance. Other agencies that contributed financial support include the Shawnee National Forest (SNF) and the Illinois Department of Transportation (IDOT). Many thanks to Mike Spanel (SNF) and Rich Nowack (IDOT) for their assistance. Several agencies cooperated in other ways; we are. grateful to: Illinois Department of Conservation (IDOC); Joan Bade of the Monroe-Randolph Bi- County Health Department; Russell Graham and Jim Oliver, Illinois State Museum (ISM); Dr. J. E. McPherson, Zoology Department, Southern Illinois University at Carbondale (SIUC). Further contributions were made by the National Speleological Society, Little Egypt and Mark Twain Grottoes of the National Speleological Society, and the Missouri Speleological Survey. -
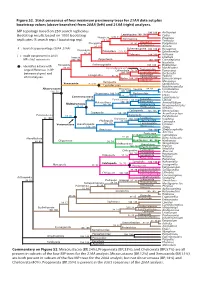
Figure S2. Strict Consensus of Four Maximum Parsimony Trees for 21AA Data Set Plus Bootstrap Values (Above Branches) from 20AA (Left) and 21AA (Right) Analyses
Figure S2. Strict consensus of four maximum parsimony trees for 21AA data set plus bootstrap values (above branches) from 20AA (left) and 21AA (right) analyses. MP topology based on 250 search replicates. 100 100 Antheraea Bootstrap results based on 1000 bootstrap Lepidoptera 100 100 Cydia Neoptera [23] 44 Dermaptera Prodoxus replicates (5 search reps / bootstrap rep). 68 66 Forficula Blattodea Pterygota 92 96 Periplaneta Orthoptera 96 97 Acheta # : bootstrap percentage (20AA 21AA) Ephemeroptera Dicondylia 100 100 Hexagenia Paleoptera [37] 52 Ephemerella 98 98 Odonata 100 100 Ischnura [ ] : node not present in 20AA Insecta Libellula MP strict consensus 100 100 Zygentoma 100 100 Ctenolepisma Nicoletia Archaeognatha : Identifies 6 taxa with Hexapoda 100 100 Pedetontus 89 86 Machiloides Entomobryomorpha Tomoceridae large differences in BP Collembola 70 76 Tomocerus between degen1 and 100 100 Entomobryidae Orchesella Entognatha 82 82 Poduridae Podura 20AA analyses. Diplura 100 100 Campodeidae Japygidae Eumesocampa Remipedia Metajapyx Xenocarida [31] 51 Speleonectes Cephalocarida Hutchinsoniella Altocrustacea Thoracica Sessilia 88 83 Semibalanus [14] 31 100 100 Chthamalus Thecostraca 100 100 Pedunculata Lepas Communostraca Rhizocephala Loxothylacus 96 89 Eumalacostraca 84 85 Eucarida Libinia Malacostraca 100 100 Peracarida Armadillidium Multicrustacea 100 100 Hoplocarida 69 84 Neogonodactylus Phyllocarida Nebalia Cyclopoida 100 100 Mesocyclops Copepoda 100 100 Acanthocyclops Pancrustacea 65 82 Calanoida Eurytemora 96 100 Diplostraca 100 100 -

Millipedes and Centipedes? Millipedes and Centipedes Are Both Arthropods in the Subphylum Myriapoda Meaning Many Legs
A Teacher’s Resource Guide to Millipedes & Centipedes Compiled by Eric Gordon What are millipedes and centipedes? Millipedes and centipedes are both arthropods in the subphylum Myriapoda meaning many legs. Although related to insects or “bugs”, they are not actually insects, which generally have six legs. How can you tell the difference between millipedes and centipedes? Millipedes have two legs per body segment and are typically have a body shaped like a cylinder or rod. Centipedes have one leg per body segment and their bodies are often flat. Do millipedes really have a thousand legs? No. Millipedes do not have a thousand legs nor do all centipedes have a hundred legs despite their names. Most millipedes have from 40-400 legs with the maximum number of legs reaching 750. No centipede has exactly 100 legs (50 pairs) since centipedes always have an odd number of pairs of legs. Most centipedes have from 30- 50 legs with one order of centipedes (Geophilomorpha) always having much more legs reaching up to 350 legs. Why do millipedes and centipedes have so many legs? Millipedes and centipedes are metameric animals, meaning that their body is divided into segments most of which are completely identical. Metamerization is an important phenomenon in evolution and even humans have a remnant of former metamerization in the repeating spinal discs of our backbone. Insects are thought to have evolved from metameric animals after specializing body segments for specific functions such as the head for sensation and the thorax for locomotion. Millipedes and centipedes may be evolutionary relatives to the ancestor of insects and crustaceans. -

A Review of the Millipede Genus Sinocallipus Zhang, 1993 (Diplopoda, Callipodida, Sinocallipodidae), with Notes on Gonopods Monotony Vs
A peer-reviewed open-access journal ZooKeys 90: 13–34 (2011) Review of genus Sinocallipus 13 doi: 10.3897/zookeys.90.1291 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A review of the millipede genus Sinocallipus Zhang, 1993 (Diplopoda, Callipodida, Sinocallipodidae), with notes on gonopods monotony vs. peripheral diversity in millipedes Pavel Stoev1,†, Henrik Enghoff 2,‡ 1 National Museum of Natural History, 1, Tsar Osvoboditel Blvd, 1000 Sofi a and Pensoft Publishers, 13a, Geo Milev Str., 1111 Sofi a, Bulgaria 2 Natural History Museum of Denmark (Zoological Museum), University of Copenhagen, Universitetsparken 15, DK-2100 København Ø, Denmark † urn:lsid:zoobank.org:author:333ECF33-329C-4BC2-BD6A-8D98F6E340D4 ‡ urn:lsid:zoobank.org:author:9B9D901F-D6C8-4BCA-B11B-CF6EE85B16DC Corresponding author : Pavel Stoev ( [email protected] ) Academic editor: Robert Mesibov | Received 21 March 2011 | Accepted 29 March 2011 | Published 14 April 2011 urn:lsid:zoobank.org:pub:A2B3DB26-59B7-4C7C-947E-4782EF5CF648 Citation: Stoev P, Enghoff H (2011) A review of the millipede genus Sinocallipus Zhang, 1993 (Diplopoda, Callipodida, Sinocallipodidae), with notes on gonopods monotony vs. peripheral diversity in millipedes. ZooKeys 90 : 13 – 34 . doi: 10.3897/zookeys.90.1291 Abstract Th e millipede genus Sinocallipus is reviewed, with four new cave-dwelling species, S. catba, S. deharvengi, S. jaegeri and S. steineri, being described from caves in Laos and Vietnam. With the new records the num- ber of species in the genus reaches six and the genus range is extended to Central Vietnam and North and Central Laos. Both, S. jaegeri from Khammouan Province in Laos and S. -

Ordinal-Level Phylogenomics of the Arthropod Class
Ordinal-Level Phylogenomics of the Arthropod Class Diplopoda (Millipedes) Based on an Analysis of 221 Nuclear Protein-Coding Loci Generated Using Next- Generation Sequence Analyses Michael S. Brewer1,2*, Jason E. Bond3 1 Department of Environmental Science, Policy, and Management, University of California Berkeley, Berkeley, California, United States of America, 2 Department of Biology, East Carolina University, Greenville, North Carolina, United States of America, 3 Department of Biological Sciences and Auburn University Museum of Natural History, Auburn University, Auburn, Alabama, United States of America Abstract Background: The ancient and diverse, yet understudied arthropod class Diplopoda, the millipedes, has a muddled taxonomic history. Despite having a cosmopolitan distribution and a number of unique and interesting characteristics, the group has received relatively little attention; interest in millipede systematics is low compared to taxa of comparable diversity. The existing classification of the group comprises 16 orders. Past attempts to reconstruct millipede phylogenies have suffered from a paucity of characters and included too few taxa to confidently resolve relationships and make formal nomenclatural changes. Herein, we reconstruct an ordinal-level phylogeny for the class Diplopoda using the largest character set ever assembled for the group. Methods: Transcriptomic sequences were obtained from exemplar taxa representing much of the diversity of millipede orders using second-generation (i.e., next-generation or high-throughput) sequencing. These data were subject to rigorous orthology selection and phylogenetic dataset optimization and then used to reconstruct phylogenies employing Bayesian inference and maximum likelihood optimality criteria. Ancestral reconstructions of sperm transfer appendage development (gonopods), presence of lateral defense secretion pores (ozopores), and presence of spinnerets were considered.