Patent Application Publication ( 10) Pub
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Acla
[CANCER RESEARCH 49. 4357-4362, August 1. 1989] Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Aclarubicin Hydrochloride 1omonimi Ichihara,1 Kiyoshi Sakamoto, Katsutaka Mori, and Masanobu Akagi Department of Surgery II, Kumamoto University Medical School, Kumamoto 860, Japan 4BSTRACT MATERIALS AND METHODS Transcatheter arterial Chemoembolization therapy using polylactic Preparation of PLA-ACRms acid microspheres containing aclarubicin hydrochloride (ACR) was per PLA-ACRms were prepared in the pharmacy of the Kumamoto formed in 62 patients with primary hepatocellular carcinoma. These microspheres were about 200 ¡anin diameter and contained 10% (w/w) University Hospital. Briefly, aclarubicin hydrochloride and isopropyl aclarubicin. A single dose of polylactic acid microspheres containing ACR myristate, a medium-chain fatty acid ester, were dissolved in 7.5% (50-100 mg of ACR) was administered 1 to 8 times with a mean of 2.2 polylactic acid-methylene chloride. The resultant solution was dispersed in 1% gelatin solution and sterilized in an autoclave for 20 min at doses (a total of 160 treatments) in 62 patients. Antitumor effects were 120°C;thismixture was then stirred with a magnetic stirrer at 500 rpm observed from the decrease in serum a-fetoprotein levels (82.1% of the patients) and in two dimensional size of tumor on computed tomography for 1 h. The resultant microspheres were collected by filtration through (93.6%). The cumulative survival rate was 54.3% at 1 year, 24.6% at 2 a membrane filter (3 //m pore diameter), rinsed 2 to 3 times (in 1 liter years, and 19.2% at 3 years, respectively, among 59 patients with of distilled water), and dried under reduced pressure for 5 days. -

Us 8530498 B1 3
USOO853 0498B1 (12) UnitedO States Patent (10) Patent No.: US 8,530,498 B1 Zeldis (45) Date of Patent: *Sep. 10, 2013 (54) METHODS FORTREATING MULTIPLE 5,639,476 A 6/1997 OShlack et al. MYELOMAWITH 5,674,533 A 10, 1997 Santus et al. 3-(4-AMINO-1-OXO-1,3-DIHYDROISOINDOL- 395 A 22 N. 2-YL)PIPERIDINE-2,6-DIONE 5,731,325 A 3/1998 Andrulis, Jr. et al. 5,733,566 A 3, 1998 Lewis (71) Applicant: Celgene Corporation, Summit, NJ (US) 5,798.368 A 8, 1998 Muller et al. 5,874.448 A 2f1999 Muller et al. (72) Inventor: Jerome B. Zeldis, Princeton, NJ (US) 5,877,200 A 3, 1999 Muller 5,929,117 A 7/1999 Muller et al. 5,955,476 A 9, 1999 Muller et al. (73) Assignee: Celgene Corporation, Summit, NJ (US) 6,020,358 A 2/2000 Muller et al. - 6,071,948 A 6/2000 D'Amato (*) Notice: Subject to any disclaimer, the term of this 6,114,355 A 9, 2000 D'Amato patent is extended or adjusted under 35 SS f 1939. All et al. U.S.C. 154(b) by 0 days. 6,235,756 B1 5/2001 D'Amatoreen et al. This patent is Subject to a terminal dis- 6,281.230 B1 8/2001 Muller et al. claimer 6,316,471 B1 1 1/2001 Muller et al. 6,326,388 B1 12/2001 Man et al. 6,335,349 B1 1/2002 Muller et al. (21) Appl. No.: 13/858,708 6,380.239 B1 4/2002 Muller et al. -

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

Isolation and Characterization of a Chinese Hamster Ovary Cell Line Resistant to Bifunctional Nitrogen Mustards1
[CANCER RESEARCH 46, 6290-6294, December 1986] Isolation and Characterization of a Chinese Hamster Ovary Cell Line Resistant to Bifunctional Nitrogen Mustards1 Craig N. Robson, Janice Alexander, Adrian L. Harris, and Ian D. Hickson2 Departmem of Clinical Oncology, University of Newcastle upon Tyne, The Royal Victoria Infirmary, Newcastle upon Tyne, NEI 4LP, United Kingdom ABSTRACT mustards, is collaterally sensitive to nitrosoureas (10), despite the fact that both these classes of agent generate DNA inter- A drug-resistant derivative of a Chinese hamster ovary cell line has strand cross-links. been generated by chronic exposure to progressively higher concentra Although chlorambucil is widely used in the curative therapy tions of chlorambucil. The cells exhibit greater than 20-fold resistance of Hodgkin's disease (11), in modified cyclophosphamide-meth- to the cytotoxic effects of chlorambucil and comparable levels of cross- resistance to mechlorethamine and melphalan. These drugs all belong to otrexate-5-fluorouracil protocols for breast cancer (12), in ovar a class of bifunctional alkylating agents which generate DNA cross-links ian cancer (13), and in small cell lung cancer (14), cell lines by reaction at the N-7 position of guanine. However, no resistance is isolated on the basis of chlorambucil resistance have rarely been observed to several other drugs which possess a similar mechanism of reported (15). action, to en-platinum (Mamminedichloride or to bischloroethylnitrosou- Here, we describe the isolation of a CHO3 cell line which rea and mitomycin C, which cross-link DNA via the O6 position of exhibits elevated levels of resistance to the cytotoxic effects of guaninc. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Towards the Reduction of Occupational Exposure To
TOWARDS THE REDUCTION OF OCCUPATIONAL EXPOSURE TO CYTOTOXIC DRUGS by Cristian Vasile Barzan B.Sc. Simon Fraser University, 2007 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE in The Faculty of Graduate Studies (Occupational and Environmental Hygiene) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2010 © Cristian Vasile Barzan, 2010 Abstract Background : One of the most powerful and widely used techniques in cancer treatment is the use of cytotoxic drugs in chemotherapy. These drugs are inherently hazardous with many of them causing carcinogenic, mutagenic or teratogenic health outcomes. Occupational exposure to cytotoxic drugs is of great concern due to their lack of selectivity between healthy and unhealthy cells. Widespread cytotoxic drug contamination has been reported in North America, Europe and Australia. Current cleaning protocols for hazardous antineoplastic drugs include the use of disinfectants and oxidizing agents, such as household bleach. Aim : The thesis project focused on two objectives: 1) hypothesize and confirm potential hazardous by- products arising from cleaning cyclophosphamide, a widely used cytotoxic drugs, with household bleach, a commonly used cleaning agent; 2) develop an effective and safe cleaning agent for cytotoxic drugs in order to prevent and eliminate exposure to these drugs. Methods : The gas chromatograph mass spectrum (GC/MS) was used to analyze the decomposition of cyclophosphamide by household bleach (5.25% hypochlorite). The reaction was conducted in a test-tube and the by-products extracted and derivatized prior to analysis. Multiple cleaning agent compositions were tested on 10x10cm stainless steel plates spiked with the two model cytotoxic drugs, cyclophosphamide and methotrexate. -

Functional Similarity of Anticancer Drugs by MTT Bioassay
cer Scien an ce C & f o T l h a e Hiwasa et al., J Cancer Sci Ther 2011, 3.10 n r a Journal of r p u 1000099 y o J DOI: 10.4172/1948-5956. ISSN: 1948-5956 Cancer Science & Therapy Rapid Communication Open Access Functional Similarity of Anticancer Drugs by MTT Bioassay Takaki Hiwasa1*,Takanobu Utsumi1, Mari Yasuraoka1, Nana Hanamura1, Hideaki Shimada2, Hiroshi Nakajima3, Motoo Kitagawa4, Yasuo Iwadate4,5, Ken-ichiro Goto6, Atsushi Takeda7, Kenzo Ohtsuka8, Hiroyoshi Ariga9 and Masaki Takiguchi1 1Department of Biochemistry, and Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 2Department of Surgery, School of Medicine, Toho University, Ota-ku, Tokyo 143-8541, Japan 3Department of Molecular Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 4Department of Molecular and Tumor Pathology, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 5Department of Neurological Surgery, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 6Department of Orthopaedic Surgery, National Hospital Organization, Shimoshizu Hospital, Yotsukaido, Chiba 284-0003, Japan 7Laboratory of Biochemistry, Graduate School of Nutritional Sciences, Sagami Women’s University, Sagamihara, Kanagawa 252-0383, Japan 8Laboratory of Cell & Stress Biology, Department of Environmental Biology, Chubu University, Matsumoto-cho, Kasugai, Aichi 487-8501, Japan 9Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo 060-0812, Japan Abstract We prepared normal or Ha-ras-transformed NIH3T3 cells transfected stably or transiently with various tumor- related genes. The chemosensitivity of the transfected clones to 16 anticancer drugs was compared to the parental control cells using the MTT assay. -

Metabolic Carbonyl Reduction of Anthracyclines — Role in Cardiotoxicity and Cancer Resistance
Invest New Drugs DOI 10.1007/s10637-017-0443-2 REVIEW Metabolic carbonyl reduction of anthracyclines — role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents Kamil Piska1 & Paulina Koczurkiewicz1 & Adam Bucki 2 & Katarzyna Wójcik-Pszczoła1 & Marcin Kołaczkowski2 & Elżbieta Pękala1 Received: 23 November 2016 /Accepted: 17 February 2017 # The Author(s) 2017. This article is published with open access at Springerlink.com Summary Anthracycline antibiotics (ANT), such as doxoru- monoHER, curcumin, (−)-epigallocatechin gallate, resvera- bicin or daunorubicin, are a class of anticancer drugs that are trol, berberine or pixantrone, and their modulating effect on widely used in oncology. Although highly effective in cancer the activity of ANT is characterized and discussed as potential therapy, their usefulness is greatly limited by their mechanism of action for novel therapeutics in cancer cardiotoxicity. Possible mechanisms of ANT cardiotoxicity treatment. include their conversion to secondary alcohol metabolites (i.e. doxorubicinol, daunorubicinol) catalyzed by carbonyl re- Keywords Anthracyclines . Cardiotoxicity . Resistance . ductases (CBR) and aldo-keto reductases (AKR). These me- Pharmacokinetics . Drug metabolism . Anticancer agents tabolites are suspected to be more cardiotoxic than their parent compounds. Moreover, overexpression of ANT-reducing en- zymes (CBR and AKR) are found in many ANT-resistant Introduction cancers. The secondary metabolites show decreased cytotoxic properties and are more susceptible to ABC-mediated efflux Anthracyclines (ANT) are a class of cell-cycle non-specific than their parent compounds; thus, metabolite formation is anticancer antibiotics that were first isolated from the considered one of the mechanisms of cancer resistance. Streptomyces genus in the early 1960s. -

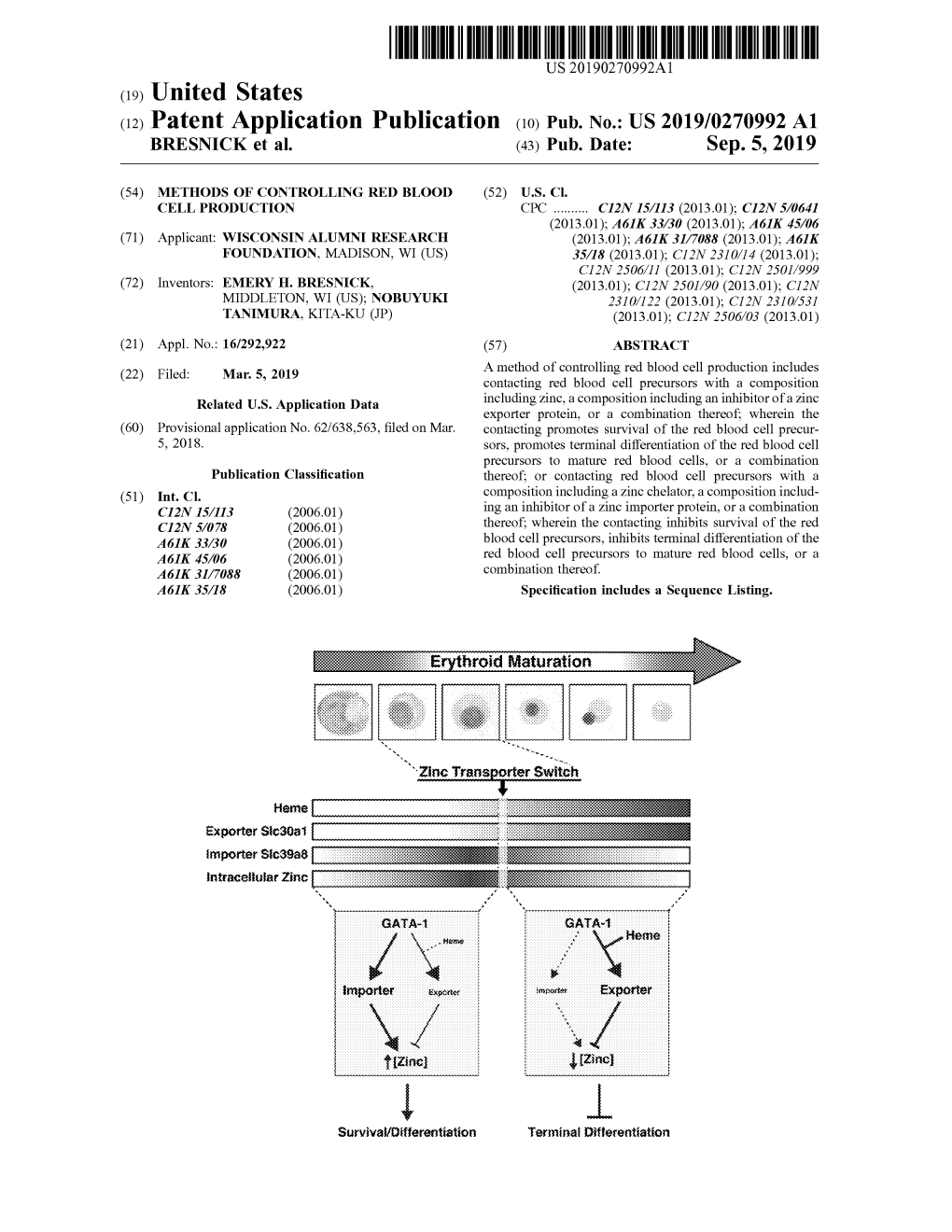
C19) United States 02) Patent Application Publication (10) Pub
1111111111111111 IIIIII IIIII 111111111111111 111111111111111 IIIII IIIII IIIII 1111111111 11111111 US 20190241665Al c19) United States 02) Patent Application Publication (10) Pub. No.: US 2019/0241665 Al KREEGER et al. (43) Pub. Date: Aug. 8, 2019 (54) METHODS OF INHIBITING METASTASIS IN C07K 16/24 (2006.01) CANCER C12N 15/113 (2006.01) A61K 31/439 (2006.01) (71) Applicant: WISCONSIN ALUMNI RESEARCH (52) U.S. Cl. FOUNDATION, Madison, WI (US) CPC .......... C07K 1612854 (2013.01); A61P 35/04 (2018.01); C07K 16/24 (2013.01); Cl2N (72) Inventors: PAMELA KAY KREEGER, 2310/14 (2013.01); C12N 15/1138 (2013.01); MIDDLETON, WI (US); MOLLY A61K 31/439 (2013.01); C07K 2317/76 JANE CARROLL, MADISON, WI (2013.01); C07K 16/2866 (2013.01) (US); KAITLIN C. FOGG, FITCHBURG, WI (US) (57) ABSTRACT (21) Appl. No.: 16/256,065 As described herein, a method of inhibiting metastasis in (22) Filed: Jan. 24, 2019 cancer includes administering to a human subject diagnosed with a cancer of an organ of the peritoneal cavity a thera Related U.S. Application Data peutically effective amount of an inhibitor of CCR5 or P-selectin. Preferably the subject has a tumor positive for a (60) Provisional application No. 62/621,769, filed on Jan. ligand of P-selectin such as a CD24+ or PSGL-1 + tumor. 25, 2018. Analysis of samples from HGSOC patients confirmed increased MIP-1 fJ and P-selectin, suggesting that this novel Publication Classification multi-cellular mechanism can be targeted to slow or stop (51) Int. Cl. metastasis in cancers such as high-grade serous ovarian C07K 16/28 (2006.01) cancer, for example by using anti-CCR5 and P-selectin A61P 35/04 (2006.01) therapies developed for other indications. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2006) – Supplement 1 (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2006) – Supplement 1 (Rev. 1) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABACAVIR 136470-78-5 ACEXAMIC ACID 57-08-9 ABAFUNGIN 129639-79-8 ACICLOVIR 59277-89-3 ABAMECTIN 65195-55-3 ACIFRAN 72420-38-3 ABANOQUIL 90402-40-7 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABIRATERONE 154229-19-3 ACIVICIN 42228-92-2 ABITESARTAN 137882-98-5 ACLANTATE 39633-62-0 ABLUKAST 96566-25-5 ACLARUBICIN 57576-44-0 ABUNIDAZOLE 91017-58-2 ACLATONIUM NAPADISILATE 55077-30-0 ACADESINE 2627-69-2 ACODAZOLE 79152-85-5 ACAMPROSATE 77337-76-9 ACONIAZIDE 13410-86-1 ACAPRAZINE 55485-20-6 ACOXATRINE 748-44-7 ACARBOSE 56180-94-0 ACREOZAST 123548-56-1 ACEBROCHOL 514-50-1 ACRIDOREX 47487-22-9 ACEBURIC -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9