Chapter 1. the Properties of Gases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

[email protected]
Fundamentals of mechanical engineering [email protected] MOHAMMED ABASS ALI What is thermodynamics Thermodynamics is the branch of physics that studies the effects of temperature and heat on physical systems at the macroscopic scale. In addition, it also studies the relationship that exists between heat, work and energy. Thermal energy is found in many forms in today’s society including power generation of electricity using gas, coal or nuclear, heating water by gas or electric. THE BASICS OF THERMODYNAMICS Basic concepts Properties are Features of a system which include mass, volume, energy, pressure and temperature. Thermodynamics also considers other quantities that are not physical properties, such as mass flow rates and energy transfers by work and heat. Energy forms Fluids and solids can possess several forms of energy. All fluids possess energy due to their temperature and this is referred to as ‘internal energy’. They will also possess ‘ potential energy’ (PE) due to distance (z) above a datum level and if the fluid is moving at a velocity (v), it will also possess ‘kinetic energy’. If the fluid is pressurised, it will possess ‘flow energy’ (FE). Pressure and temperature are the two governing factors and internal energy can be added to FE to produce a single property called ‘enthalpy’. Internal energy The molecules of a fluid possess both kinetic energy (KE) and PE relative to an internal datum. Generally, this is regarded simply as the energy due to its temperature and the change in internal energy in a fluid that undergoes a temperature change is given by ΔU = mcΔT The total internal energy is denoted by the symbol ‘U’, which has values of J, kJ or MJ; also the specific internal energy ‘u’ has the values of kJ/kg. -

Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014
Unit 5 Interactive Notebook Gas Laws and Kinetic Molecular Theory Grant Union High School January 6, 2014 – January 29, 2014 Student Mastery Scale of Learning Goals Date Page Std Learning Goal Homework Mastery 1/6/14 1/7/14 1/8/14 1/9/14 1/10/14 1/13/14 1/14/14 1/15/14 1 1/16/14 1/21/14 1/22/14 1/23/14 1/24/14 1/27/14 1/28/14 1/29/14 Unit 5 EXAM 2 California Standard Gas Laws 4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept: a. Students know the random motion of molecules and their collisions with a surface create the observable pressure on that surface. Fluids, gases or liquids, consist of molecules that freely move past each other in random directions. Intermolecular forces hold the atoms or molecules in liquids close to each other. Gases consist of tiny particles, either atoms or molecules, spaced far apart from each other and free to move at high speeds. Pressure is defined as force per unit area. The force in fluids comes from collisions of atoms or molecules with the walls of a container. Air pressure is created by the weight of the gas in the atmosphere striking surfaces. Gravity pulls air molecules toward Earth, the surface that they strike. Water pressure can be understood in the same fashion, but the pressures are much greater because of the greater density of water. -

Fundamentals of Hypersonic Flow - Aerothermodynamics
FUNDAMENTALSOFHYPERSONICFLOW- AEROTHERMODYNAMICS D. G. Fletcher von Karman Institute, Belgium 1. Introduction To aid in understanding the different topics discussed in the current Lecture Series and to provide background material for interested participants who may not have specialized training in this field, a brief summary of the fundamental attributes of hypersonic flows is given. Many of the topics that are introduced in this section will be elaborated further in contributions related to specific subjects related to sustained hypersonic flight. The differencesbetweenthethermalandchemicalaspectsofhypersonicflowandsupersonic flow are therefore highlighted. The age of some of the figures used in the subsequent discussion reflect the fact that the problems of hypersonic flight are not newly discovered! Fig. 1.1 Flight trajectories for different hypersonic vehicles comparing sustained atmo- spheric flight with re-entry. The hypersonic flight regime includes atmospheric entry and re-entry, ground testing, and flight for both powered and unpowered vehicles. In the present Lecture Series, the main interest is on sustained and controlled hypersonic flight, whether for military or civil transport application. Even though it is not currently certified for flight, there is one operational hypersonic vehicle: the space shuttle of NASA. At least 20 years before the development of the shuttle a significant activity in hypersonic flight research was conducted by the US Air Force in their X-15 program. This vehicle has reached a flight Mach number of 6.7 on its final flight, which also used to test a hypersonic ramjet engine. Direct shock impingement on the pylon holding a dummy engine caused severe heating and structural damage, and this was one of many lessons learned from the program. -

The Perfect Gas
LANDMARK UNIVERSITY, OMU-ARAN LECTURE NOTE 2 COLLEGE: COLLEGE OF SCIENCE AND ENGINEERING DEPARTMENT: MECHANICAL ENGINEERING ALPHA 2016-17 ENGR. ALIYU, S.J. Course code: MCE 211 Course title: INTRODUCTION TO MECHANICAL ENGINEERING. Course Units: 2 UNITS. Course status: compulsory THE PERFECT GAS. The characteristic equation of state. At temperatures that are considerably in excess of the critical temperature of a fluid, and also at very low pressures, the vapour of the fluid tends to obey the equation; . No gases in practice obey this law rigidly, but many gases tend towards it. An imaginary ideal gas which obey the law is called a perfect gas, and the equation , pv/T = R, is called the characteristic of state of a perfect gas. The constant R, is called the gas constant. The unit of R are N m/kg K or kJ/kg K. Each perfect gas has a different gas constant. The characteristic equation is usually written; pv = RT……1 or for m kg. occupying V m3, pV = mRT ……………..2 Another form of the characteristic equation can be derived using the kilogram-mole as a unit. The kilogram-mole is defined as a quantity of gas equivalent to M kg. of the gas, where M is the molecular weight of the gas (e.g since the molecular weight of oxygen is 32, then 1 kg. mole of oxygen is equivalent 32 kg of oxygen). From the definition of kilogram-mole, for m kg of a gas we have, m = nM ………………3 (where n is the number of moles) Note: Since the standard of mass is the kg. -
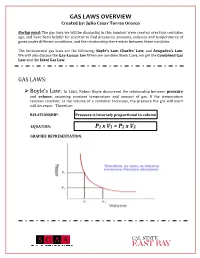
GAS LAWS OVERVIEW Created By: Julio Cesar Torres Orozco
GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco Background: The gas laws we will be discussing in this handout were created over four centuries ago, and have been helpful for scientist to find pressures, amounts, volumes and temperatures of gases under different conditions, and the relationship there exists between these variables. The fundamental gas laws are the following: Boyle’s Law, Charles’ Law, and Avogadro’s Law. We will also discuss the Gay-Lussac law When we combine these Laws, we get the Combined Gas Law and the Ideal Gas Law. GAS LAWS: ! Boyle’s Law: In 1662, Robert Boyle discovered the relationship between pressure and volume, assuming constant temperature and amount of gas. If the temperature remains constant, as the volume of a container increases, the pressure the gas will exert will decrease. Therefore: RELATIONSHIP: Pressure is inversely proportional to volume EQUATION: P1 x V1 = P2 x V2 GRAPHIC REPRESENTATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco ! Charles’ Law: In 1787, Jacques Charles discovered how temperature and volume are related, assuming that the amount of gas and pressure are constant. An increase in temperature will also increase the volume of the gas. Therefore: RELATIONSHIP: Volume is directly proportional to temperature EQUATION: GRAPHIC REPRESENTATION: ! Gay-Lussacs’ Law: This law shows the relationship there exists between temperature and pressure of gasses. Given a constant volume, if the temperature increases, the pressure will also increase. Therefore: RELATIONSHIP: Pressure is directly proportional to temperature EQUATION: GAS LAWS OVERVIEW Created by: Julio Cesar Torres Orozco GRAPHIC REPRESENTATION: ! Avogadro’s Law: In 1811, Amedeo Avogadro was able to identify the correlation between the amount of gas (n) and its volume, assuming that temperature and pressure are constant. -

4 Pressure and Viscosity
4 Pressure and Viscosity Reading: Ryden, chapter 2; Shu, chapter 4 4.1 Specific heats and the adiabatic index First law of thermodynamics (energy conservation): d = −P dV + dq =) dq = d + P dV; (28) − V ≡ ρ 1 = specific volume [ cm3 g−1] dq ≡ T ds = heat change per unit mass [ erg g−1] − s ≡ specific entropy [ erg g−1 K 1]: The specific heat at constant volume, @q −1 −1 cV ≡ [ erg g K ] (29) @T V is the amount of heat that must be added to raise temperature of 1 g of gas by 1 K. At constant volume, dq = d, and if depends only on temperature (not density), (V; T ) = (T ), then @q @ d cV ≡ = = : @T V @T V dT implying dq = cV dT + P dV: 1 If a gas has temperature T , then each degree of freedom that can be excited has energy 2 kT . (This is the equipartition theorem of classical statistical mechanics.) The pressure 1 ρ P = ρhjw~j2i = kT 3 m since 1 1 3kT h mw2i = kT =) hjw~j2i = : 2 i 2 m Therefore kT k P V = =) P dV = dT: m m Using dq = cV dT + P dV , the specific heat at constant pressure is @q dV k cP ≡ = cV + P = cV + : @T P dT m Changing the temperature at constant pressure requires more heat than at constant volume because some of the energy goes into P dV work. 19 For reasons that will soon become evident, the quantity γ ≡ cP =cV is called the adiabatic index. A monatomic gas has 3 degrees of freedom (translation), so 3 kT 3 k 5 k 5 = =) c = =) c = =) γ = : 2 m V 2 m P 2 m 3 A diatomic gas has 2 additional degrees of freedom (rotation), so cV = 5k=2m, γ = 7=5. -

The Gas Laws A. Introduction. 1. Comparison of Three States of Matter
CHAPTER FIVE: THE GASEOUS STATE Part One: The Gas Laws A. Introduction. 1. Comparison of three states of matter: solids condensed states liquids (high density, hard to compress) fluids (flow freely) gases (low density, easy to compress) 1 mole liquid H2O occupies 18 mL 1 mole H2O vapor at 100° C and atmospheric pressure occupies 30,600mL Thus, gas molecules must be far apart compared to molecular sizes and interact only weakly. 2. Composition of dry air by volume: 78% N2, 21% O2, 1% Ar, traces of other. 3. Properties of gases: a. Easily compressed into small volumes by applying pressure. b. Exert a pressure P on their surroundings; an equal pressure must be applied to confine them. c. Expand without limit to uniformly and completely occupy the volume of any container. d. Individual molecules exhibit a chaotic motion called diffusion. e. Properties described by gas laws. Show them “A Little Box of Air.” Chapter 5 Page 1 B. Pressure (P). (Section 5.1) 1. P = force per unit area produced by incessant collisions of particles with container walls. 2. Measurement of atmospheric pressure (Torricelli barometer): h ∝ pressure average height h: = 760 mm Hg at sea level P = 760 mm Hg = 1 atmosphere (atm) ≈ 30 inches 1 mm Hg = 1 “torr” SI unit of P is the pascal (Pa) 760 mm Hg: = 1 atm = 1.01325 x 105 Pa = 101.325kPa 3. Pressure of a column of liquid = hydrostatic pressure: P = gdh = accel. of gravity x density of liquid x height of column 2 g=9.81 m/s Chapter 5 Page 2 4. -

AA210A Fundamentals of Compressible Flow
AA210A Fundamentals of Compressible Flow Chapter 2 -Thermodynamics of dilute gases 9/29/20 1 2.1 Introduction The power of thermodynamics comes from the fact that the change in the state of a fluid is independent of the actual physical process by which the change is achieved; thermodynamic theory is expressed in terms of perfect differentials. 2.2 Thermodynamics Piston-cylinder combination. First law of thermodynamics. 9/29/20 2 The work done by the system is the mechanical work by a force acting over a distance. When dealing with fluid flows it is convenient to work in terms of intensive (per unit mass) variables. If there is an equation of state for the substance inside the cylinder the first law is 9/29/20 3 According to Pfaff’s theorem there must exist an integrating factor such that the first law becomes a perfect differential. Once one accepts the first law and the existence of an equation of state then two new variables of state are implied; an integrating factor, the temperature, and an associated integral called entropy. The final result is the famous Gibbs equation which is the starting point for the field of thermodynamics The partial derivatives of the entropy are 9/29/20 4 2.3 The Carnot Cycle 9/29/20 5 Thermodynamic efficiency of the cycle First Law Over the cycle the change in internal energy is zero and the work done is So the efficiency is Since the temperature is constant during the heat interaction δq Q Q ds = = 1 + 2 = 0 Finally !∫ !∫ T T1 T2 9/29/20 6 2.3.1 The absolute scale of temperature For any Carnot cycle regardless of the working fluid This relation enables an absolute scale of temperature to be defined that is independent of the properties of any particular substance. -

Chapter 14: Gases
418-451_Ch14-866418 5/9/06 6:19 PM Page 418 CHAPTER 14 Gases Chemistry 2.a, 3.c, 3.d, 3.e, 4.a, 4.c, 4.d, 4.e, 4.f, 4.g, 4.h I&E 1.b, 1.c, 1.d What You’ll Learn ▲ You will use gas laws to cal- culate how pressure, tem- perature, volume, and number of moles of a gas will change when one or more of these variables is altered. ▲ You will compare properties of real and ideal gases. ▲ You will apply the gas laws and Avogadro’s principle to chemical equations. Why It’s Important From barbecuing on a gas grill to taking a ride in a hot-air balloon, many activities involve gases. It is important to be able to predict what effect changes in pressure, temperature, volume, or amount, will have on the properties and behavior of a gas. Visit the Glencoe Chemistry Web site at chemistrymc.com to find links about gases. Firefighters breathe air that has been compressed into tanks that they can wear on their backs. 418 Chapter 14 418-451_Ch14-866418 5/9/06 6:19 PM Page 419 DISCOVERY LAB More Than Just Hot Air Chemistry 4.a, 4.c I&E 1.d ow does a temperature change affect the air in Ha balloon? Safety Precautions Always wear goggles to protect eyes from broken balloons. Procedure 1. Inflate a round balloon and tie it closed. 2. Fill the bucket about half full of cold water and add ice. 3. Use a string to measure the circumference of the balloon. -

On Classical Ideal Gases
Entropy 2013, 15, 960-971; doi:10.3390/e15030960 OPEN ACCESS entropy ISSN 1099-4300 www.mdpi.com/journal/entropy Article On Classical Ideal Gases Jacques Arnaud 1, Laurent Chusseau 2;* and Fabrice Philippe 3 1 Mas Liron, F30440 Saint Martial, France; E-Mail: [email protected] (J.A.) 2 IES, UMR n◦5214 au CNRS, Université Montpellier II, F34095 Montpellier, France 3 LIRMM, UMR n◦5506 au CNRS, 161 rue Ada, F34392 Montpellier, France; E-Mail: [email protected] (F.P.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-467414-975; Fax: +33-467547-134. Received: 12 December 2012; in revised form: 15 January 2013 / Accepted: 21 February 2013 / Published: 27 February 2013 Abstract: We show that the thermodynamics of ideal gases may be derived solely from the Democritean concept of corpuscles moving in vacuum plus a principle of simplicity, namely that these laws are independent of the laws of motion, aside from the law of energy conservation. Only a single corpuscle in contact with a heat bath submitted to a z and t-invariant force is considered. Most of the end results are known but the method appears to be novel. The mathematics being elementary, the present paper should facilitate the understanding of the ideal gas law and of classical thermodynamics even though not-usually-taught concepts are being introduced. Keywords: ideal gas law; relativistic gases submitted to gravity; corpuscular concepts; classical gas theory; Democritus physics; canonical single-corpuscle thermodynamics 1. Introduction This paper is devoted to an alternative derivation of the ideal gas law, which agrees with the accepted postulates of statistical mechanics, and may present pedagogical and scientific interest. -

The Ideal and Combined Gas Laws PV = Nrt Or P1V1 = P2V2 T1 T2
The Ideal and Combined Gas Laws PV = nRT or P1V1 = P2V2 T1 T2 Use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be PV = nRT 1) If four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? 2) If I initially have a gas with a pressure of 84 kPa and a temperature of 350 C and I heat it an additional 230 degrees, what will the new pressure be? Assume the volume of the container is constant. 3) My car has an internal volume of 2600 liters. If the sun heats my car from a temperature of 200 C to a temperature of 550 C, what will the pressure inside my car be? Assume the pressure was initially 760 mm Hg. 4) How many moles of gas are in my car in problem #3? 5) A toy balloon filled with air has an internal pressure of 1.25 atm and a volume of 2.50 L. If I take the balloon to the bottom of the ocean where the pressure is 95 atmospheres, what will the new volume of the balloon be? How many moles of gas does the balloon hold? (Assume T = 285 K) For chemistry help, visit www.chemfiesta.com © 2000 Cavalcade Publishing - All Rights Reserved MIXED GAS LAWS WORKSHEET Created by Tara L. Moore at www.learning.mgccc.cc.ms.us/pk/sciencedocs/gaslawwksheet.htm Directions: Answer each question below. -

Molecular Theory of Ideal Gases March 1, 2021
1 Statistical Thermodynamics Professor Dmitry Garanin Molecular theory of ideal gases March 1, 2021 I. PREFACE Molecular theory can be considered as a preliminary to statistical physics. While the latter employs a more sophisti- cated formalism encompassing quantum-mechanical systems, the former operates with a classical ideal gas. Molecular theory studies the relation between the temperature of the gas and the kinetic energy of the molecules, pressure on the walls due to the impact of the molecules, distribution of molecules over velocities etc. All these results can be obtained in a more formal way in statistical physics. However, it is convenient to first study molecular theory at a more elementary level. On the other hand, molecular theory includes kinetics of classical gases that studies nonequilibrium phenomena such as heat conduction and diffusion (not a part of this course) II. BASIC ASSUMPTIONS OF THE MOLECULAR THEORY 1. Motion of atoms and molecules is described by classical mechanics 2. The number of particles in a considered macroscopic volume is very large. As there are about 1019 molecules in 1 cm3 at normal conditions, this assumption holds down to high vacuums. Because of the large number of particles, the impacts of individual particles on the walls merge into time-independent pressure. 3. The characteristic distance between the molecules largely exceeds the molecular size and the typical radius of intermolecular forces. This assumption allows to consider the gas as ideal, with the internal energy dominated by the kinetic energy of the molecules. In describing equilibrium properties of the ideal gas collisions between the molecules can be neglected.