RADIOACTIVITY of PROMETHEUM DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosoph
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Status of Fission Yield Measurements
nij»wiiM>iT»i»n'i»wwr I '• • «• PORTIOHS OF THIS &EPOTT ARE till •{•1& * i STATUS OF FI5.SIOM YIELD MEASUREHEMTS - i William J. Maeck Exxon Nuclear Idaho Company, Inc.* Idaho National Engineering Laboratory P.O. Box 2300 Idaho Fails, Idaho 83401, USA NOTICE . vtrpiird at Mt t*W. nm my < at i!tt$lte<K of anunwi my I*gal ! •prfn-nu Urn in uu *nuld noi | in(iuif.f privately 'IUWII n Prepared For Third ASTM-EURATOM Symposium On Reactor Dosimetry To be held at Ispra, Italy October 1-5, 1979 rUnder U^S... Department of Energy Idaho Operations Office Contract £- /L- - AC 07- 7c!XV>o 1. INTRODUCTION Fission yield data, for all neutron energies, play an important role in f many of the disiplines (fuel burnup, /ission rate measurements, dosimetry, reactor physics, damage studies, post irradiation fuel analysis, etc-) being discussed at this symposium. This paper reviews and presents a brief discussion i of the current status of fission yield measurement programs being conducted in the major laboratories throughout the world, and when possible, to—identify future activities. The contents of this paper are based on tv/o sources: 1) a mailing by the author to various principal investigators furnishing a form which could be used to describe their most current work, and 2) reference to the most recent IAEA Nuclear Data Section document "Progress in Fission Product Nuclear Data" , which is a compilation of current measurement activities relative i to fission product nuclear data, • • , The~gGnera-l-orgunizat.ij?Jl_oI._t]ie,.p.aper_Js..by-_f.issioning. -

Gjftjoh KB 06Ctivifibs
A gjftjOH KB EffBl&JS %ASI 06CTIVIfIBS IdXnmi 0SCIEEEEIQ5 thesis subs&ttea by 3BfcCBKSJUffBA OTA. H* 3©*, (University of Petna). far the degree of sector up raxes era* bepertnent of Seturel Philosophy, University of 2diobur,^i» HAY, 1950. C OB IE fl IS, Page IRTFOIJtJCTIGN • 1 SECT I OK I The JSatural Beta Activity of JBeodymium 3 "Results . » • • . 9 The effect of 3 elf-Ahsorption on the Estimate of Half life • • IS The Uncertainty in the Estimate of the Maximum Energy of f> -Particles • 16 The Isotoplo Assignment of the fieodymlum p -Activity * • 17 SECTIOB II Introduction * * « • . 30 Pleochroic Haloes . * . • 30 The Search for <k -Particles of Range about 2 cm amongst medium heavy Elements, • • . • 39 studies of Reodymium Activity with a Proportional Counter. • • 42 Experimental Results 47 Studies of -Particles from lecdymium in the Buclear Emulsion . 56 Experimental Results with nuclear w Emulsion. ♦ . • 59 Discussion ..... 63 CONTENTS (Oontd. ) Page SECTION II fContd*) ^-Activity araon^- the Ear© Earth Elements « • * * 69 isotypic Assignment • « « 76 SECTION III Studies In the Peaiosctivity of lanthanum 80 Experimental Arrangement 82 Experimental Results , ♦ * 82 Acknowledgments . , # 87 References , , • 89 £vvtsv3 efc • ^ ^<ry^c Uo-jkhs i^rc cry^jQX L (tJLc^J-Jt^ • f~ Hu = lii7 -s tlf.r' >• na. <°h 'Axa-oL AfT. ) 7«* -- 7-i2 . U- » 3,.o ^ A^L ,°/c> '«f «f- Mf J iX <vr "Vw\ <51 «v\ '. ^ ^ •=. XL. "U<* d ^NJ(, $ I v '<f«* ^■5^ ^V-0^ >5 IfO . t > . Is-' ^3 Jxwj flL «^u» ^w\jl"\ icLtxv» ^ V" twxxL-/ ^ - &\t-a-c-A*J<-A- f&Mx ~J *aaJI mU O-SV ca^U-t TLc y <~/^e)x*~* - 1 » 4 * k .31 . -
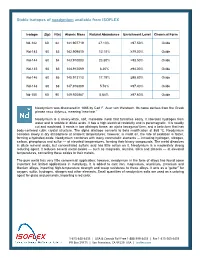
Stable Isotopes of Neodymium Available from ISOFLEX
Stable isotopes of neodymium available from ISOFLEX Isotope Z(p) N(n) Atomic Mass Natural Abundance Enrichment Level Chemical Form Nd-142 60 82 141.907719 27.13% >97.50% Oxide Nd-143 60 83 142.909810 12.18% ≥79.00% Oxide Nd-144 60 84 143.910083 23.80% >98.50% Oxide Nd-145 60 85 144.912569 8.30% ≥94.00% Oxide Nd-146 60 86 145.913113 17.19% ≥98.80% Oxide Nd-148 60 88 147.916889 5.76% ≥97.40% Oxide Nd-150 60 90 149.920887 5.64% ≥97.60% Oxide Neodymium was discovered in 1885 by Carl F. Auer von Welsbach. Its name derives from the Greek phrase neos didymos, meaning “new twin.” Neodymium is a silvery-white, soft, malleable metal that tarnishes easily. It liberates hydrogen from water and is soluble in dilute acids. It has a high electrical resistivity and is paramagnetic. It is readily cut and machined. It exists in two allotropic forms: an alpha hexagonal form, and a beta form that has body-centered cubic crystal structure. The alpha allotrope converts to beta modification at 868 ºC. Neodymium corrodes slowly in dry atmosphere at ambient temperatures; however, in moist air, the rate of oxidation is faster, forming a hydrated oxide. Neodymium combines with many nonmetallic elements — including hydrogen, nitrogen, carbon, phosphorus and sulfur — at elevated temperatures, forming their binary compounds. The metal dissolves in dilute mineral acids, but concentrated sulfuric acid has little action on it. Neodymium is a moderately strong reducing agent. It reduces several metal oxides — such as magnesia, alumina, silica and zirconia — at elevated temperatures, converting these oxides to their metals. -

Neodymium, Samarium and Europium Capture Cross-Section Adjustments Based on Ebr-Ii Integral Measurements
NEODYMIUM, SAMARIUM AND EUROPIUM CAPTURE CROSS-SECTION ADJUSTMENTS BASED ON EBR-II INTEGRAL MEASUREMENTS by R. A. Anderi and Y. D. Marker Idaho National Engineering Laboratory EG&G Idaho, Inc. F. Schmittroth Hanford Engineering Development Laboratory Contributed Paper for the NEANDC Specialist Meeting on Neutron Cross Sections of Fission-Product Nuclei December 12-14, 1979, Bologna, Italy - DISCLAIMER • DISTRIBUTION 0F THIS DOCUHSfT IS NEODYMIUM, SAMARIUM AND EUROPIUM CAPTURE CROSS-SECTION ADJUSTMENTS BASED ON EBR-II INTEGRAL MEASUREMENTS R. A. Anderl and Y. D. Harker Idaho National Engineering Laboratory EG&G Idaho, P.O. Box 1625 Idaho Falls, Idaho USA 83415 F. Schmittroth Hanford Engineering Development Laboratorty P.O. Box 1970 Richland, Washington 99352 Abstract Integral capture measurements have been made for high-enriched iso- topes of neodymium, samarium and europium irradiated in a row 3 position of EBR-II with samples located both at mid-plane and in the axial reflector. Broad response, resonance, and threshold dosimeters were included to character- ize the neutron spectra at the sample locations. The saturation reaction rates for the rare-earth samples were determined by post-irradiation mass- spectrometric analyses and for the dosimeter materials by the gamma-spectro- metric method. The HEDL maximum-likelihood analysis code, FERRET, was used to make a "least-squares adjustment" of the ENDF/B-IV rare-earth cross sections based on the measured dosimeter and fission-product reaction rates. Preliminary results to date indicate a need for a significant upward adjust- ment of the capture cross sections for ltf3Nd, li+5Nd, lt+7Sm and li+aSm. Introduction In recent years, integral data (capture reaction rates and reactivity worth measurements in fast-reactor fields) have played an important role in the evaluation of fission-product capture cross sections of importance to reactor technology, especially the development of fast reactor systems/1/. -

Neodymium Isotope Constraints on Provenance, Dispersal, and Climate‐Driven Supply of Zambezi Sediments Along the Mozam
PUBLICATIONS Geochemistry, Geophysics, Geosystems RESEARCH ARTICLE Neodymium isotope constraints on provenance, dispersal, 10.1002/2015GC006080 and climate-driven supply of Zambezi sediments along the Key Points: Mozambique Margin during the past ~45,000 years Nd isotope composition of clays deposited along the Mozambique H. J. L. van der Lubbe1,2, Martin Frank3, Rik Tjallingii4,5, and Ralph R. Schneider1 Margin Offshore deposition of Zambezi clays 1Marine Climate Research, Institute of Geosciences, University of Kiel (CAU), Germany, 2Now at Department of reflects southeast African monsoon Sedimentology and Marine Geology, Faculty of Earth and Life Sciences, Vrije Universiteit Amsterdam, Netherlands, variability 3 4 Zambezi sediment distribution on GEOMAR Helmholtz Centre for Ocean Research, Kiel, Germany, Department of Marine Geology, NIOZ - Royal the slope is also strongly affected by Netherlands Institute for Sea Research, Texel, Netherlands, 5Now at GFZ - German Research Centre for Geosciences, oceanic circulation and postglacial Potsdam, Germany sea level rise Correspondence to: Abstract Marine sediments deposited off the Zambezi River that drains a considerable part of the south- H. J. L. van der Lubbe, east African continent provide continuous records of the continental climatic and environmental conditions. [email protected] Here we present time series of neodymium (Nd) isotope signatures of the detrital sediment fraction during the past 45,000 years, to reconstruct climate-driven changes in the provenance of clays deposited along Citation: the Mozambique Margin. Coherent with the surface current regime, the Nd isotope distribution in surface van der Lubbe, H. J. L., M. Frank, R. Tjallingii, and R. R. Schneider (2016), sediments reveals mixing of the alongshore flowing Zambezi suspension load with sediments supplied by Neodymium isotope constraints on smaller rivers located further north. -

A Database of Marine and Terrestrial Radiogenic Nd and Sr Isotopes for Tracing Earth-Surface Processes Cécile L
Discussions Earth Syst. Sci. Data Discuss., https://doi.org/10.5194/essd-2018-109 Earth System Manuscript under review for journal Earth Syst. Sci. Data Science Discussion started: 8 October 2018 c Author(s) 2018. CC BY 4.0 License. Open Access Open Data A database of marine and terrestrial radiogenic Nd and Sr isotopes for tracing earth-surface processes Cécile L. Blanchet1,2 5 1Department of Geosciences, Freie Universität Berlin, Berlin, Germany 2Now at GFZ German Research Centre for Geosciences, Section 5.2 Climate Dynamics and Landscape Evolution, Potsdam, Germany Correspondence to: Cécile L. Blanchet ([email protected]) Abstract. The database presented here contains radiogenic neodymium and strontium isotope ratios measured on both 10 terrestrial and marine sediments. The main purpose of this dataset is to help assessing sediment provenance and transport processes for various time intervals. This can be achieved by either mapping sediment isotopic signature and/or fingerprinting source areas using statistical tools. The database has been built by incorporating data from the literature and the SedDB database and harmonizing the metadata, especially units and geographical coordinates. The original data were processed in three steps. Firstly, a specific attention has 15 been devoted to provide geographical coordinates to each sample in order to be able to map the data. When available, the original geographical coordinates from the reference (generally DMS coordinates) were transferred into the decimal degrees system. When coordinates were not provided, an approximate location was derived from available information in the original publication. Secondly, all samples were assigned a set of standardized criteria that help splitting the dataset in specific categories. -

Foia/Pa-2015-0050
Acknowledgements The following were the members of ICRP Committee 2 who prepared this report. J. Vennart (Chairman); W. J. Bair; L. E. Feinendegen; Mary R. Ford; A. Kaul; C. W. Mays; J. C. Nenot; B. No∈ P. V. Ramzaev; C. R. Richmond; R. C. Thompson and N. Veall. The committee wishes to record its appreciation of the substantial amount of work undertaken by N. Adams and M. C. Thorne in the collection of data and preparation of this report, and, also, to thank P. E. Morrow, a former member of the committee, for his review of the data on inhaled radionuclides, and Joan Rowley for secretarial assistance, and invaluable help with the management of the data. The dosimetric calculations were undertaken by a task group, centred at the Oak Ridge Nntinnal-..._._.__. ---...“..,-J,1 .nhnmtnrv __ac ..,.I_.,“.fnllnwr~ Mary R. Ford (Chairwoman), S. R. Bernard, L. T. Dillman, K. F. Eckerman and Sarah B. Watson. Committee 2 wishes to record its indebtedness to the task group for the completion of this exacting task. The data given in this report are to be used together with the text and dosimetric models described in Part 1 of ICRP Publication 30;’ the chapters referred to in this preface relate to that report. In order to derive values of the Annual Limit on Intake (ALI) for radioisotopes of scandium, the following assumptions have been made. In the metabolic data for scandium, a fraction 0.4 of the element in the transfer compartment is translocated to the skeleton. It is assumed thit this scandium in the skeleton is distributed between cortical bone, trabecular bone and red marrow in proportion to their respective masses. -

Late Quaternary Variability of Hydrography and Weathering Inputs on the SW Iberian Shelf from Clay Minerals and the Radiogenic I
Late Quaternary variability of hydrography and weathering inputs on the SW Iberian shelf from clay minerals and the radiogenic isotopes of neodymium, strontium and lead Dissertation zur Erlangung des Doktorgrades Dr. rer. nat. der Mathematisch-Naturwissenschaftlichen Fakultät der Christian-Albrechts-Universität zu Kiel vorgelegt von Roland Stumpf Kiel, 2011 ii Referent: Prof. Dr. Martin Frank Koreferent: Prof. Dr. Dirk Nürnberg Tag der Disputation: 24. Mai 2011 zum Druck genehmigt: 24. Mai 2011 gez., der Dekan iii iv Erklärung Hiermit versichere ich an Eides statt, dass ich diese Dissertation selbständig und nur mit Hilfe der angegebenen Quellen und Hilfsmittel erstellt habe. Weiterhin versichere ich, dass der Inhalt dieser Arbeit weder in dieser, noch in veränderter Form, einer weiteren Prüfungsbehörde vorliegt. Die Arbeit wurde unter Einhaltung der Regeln guter wissenschaftlicher Praxis der Deutschen Forschungsgemeinschaft verfasst. Kiel, den 02.05.2011 (Roland Stumpf, Dipl.‐Geol.) v vi Contents Abstract xi Kurzfassung xiii Acknowledgments xv 1. Introduction 1.1. Motivation and objectives 1 1.2. The Atlantic Meridional Overturning Circulation 3 1.3. The Mediterranean thermohaline circulation 4 1.3.1. The Mediterranean Outflow Water 5 1.3.2. Paleoceanography of MOW 6 1.4. Radiogenic isotope systems of Nd, Sr and Pb 7 1.4.1. Neodymium isotopes 8 1.4.2. Strontium isotopes 9 1.4.3. Lead isotopes 10 1.5. Clay minerals 11 2. Material, Methods & Instrumentation 2.1. Core selection and age models 13 2.2. Sample preparation 14 2.2.1. Leaching procedure 15 2.2.2. Separation of the clay‐size fraction 16 2.2.3. Preparation of XRD object slides 17 2.3. -

Download This Article in PDF Format
A&A 385, 111–130 (2002) Astronomy DOI: 10.1051/0004-6361:20020118 & c ESO 2002 Astrophysics The presence of Nd and Pr in HgMn stars L. Dolk1,G.M.Wahlgren1, H. Lundberg2,Z.S.Li2,U.Litz´en1,S.Ivarsson1, I. Ilyin3, and S. Hubrig4 1 Atomic Astrophysics, Department of Astronomy, Lund University, Box 43, 22100 Lund, Sweden 2 Department of Physics, Lund Institute of Technology, Box 118, 22100 Lund, Sweden 3 Astronomy Division, PO Box 3000, 90014 University of Oulu, Finland 4 European Southern Observatory, Casilla 19001, Santiago 19, Chile Received 11 July 2001 / Accepted 7 January 2002 Abstract. Optical region spectra for a number of upper main sequence chemically peculiar (CP) stars have been observed to study singly and doubly ionized praseodymium and neodymium lines. In order to improve existing atomic data of these elements, laboratory measurements have been carried out with the Lund VUV Fourier Transform Spectrometer (FTS). From these measurements wavelengths and hyperfine structure (hfs) have been studied for selected Pr ii,Priii and Nd iii lines of astrophysical interest. Radiative lifetimes for some excited states of Pr ii have been determined with the aid of laser spectroscopy at the Lund Laser Center (LLC) and have been combined with branching fractions measured in the laboratory to calculate gf values for some of the stronger optical lines of Pr ii. With the aid of the derived gf values and laboratory measurements of the hfs, a praseodymium abundance was derived from selected Pr ii lines in the spectrum of the Am star 32 Aqr. This abundance was used to derive astrophysical gf values for selected Pr iii lines in 32 Aqr, and these gf values were used to get a praseodymium abundance for the HgMn star HR 7775. -

Production and Separation of Terbium-149 and Terbium-152 For
AU9817402 Production and Separation of Terbium-149,152 for Targeted Cancer Therapy S SARKAR1, B J ALLEN2, S IMAM2, G GOOZEE2, J LEIGH3, H. MERIATY4 School of Medical Radiation Technology, Faculty of Health Sciences, The University of Sydney, East Street, Lidcombe2141 2St George Cancer Care Centre, Gray Street, Kogarah 2217 department of Nuclear Physics, Australian National University 4ANSTO, PMB 1, Menai 2234, NSW SUMMARY. This work concerns the production and separation of small amount of Terbium-149,152 (l49>152Tb) from natural Neodymium ("""Nd) and Praseodymium-141 (141Pr) for in-vitro studies. Carbon-12 (12C) was used as projectile to produce 149-152Tb through '42Nd(12C,5n)149Dy->I49Tb, 141Pr(12C,4n)149Tb, 142Nd(12C,2n)'52Dy->152Tb and 141Pr(12C,n)152Tb reactions. This new class of isotopes have unique physical, chemical and nuclear properties and potential applications for radionuclide therapy and diagnosis. In this study thin-target catcher foil and thick target radiochemical techniques were employed to determine the yield. Appropriate radiochemical separation techniques have been used to separate other lanthanides, from terbium, produced by secondary reactions. Optimum experimental conditions for the production of useful quantities of 149>152Tb and carrier free separation of terbium are discussed. Introduction The efficacy of radionuclides for therapy and diagnostic purposes depends on their physical, chemical and nuclear properties. Traditionally beta-emitting radionuclides such as iodine-131 (131I), yttrium-90 C°Y) and samarium-153 (I53Sm) are used in radionuclide therapy. Beta-emitting radionuclides are effective in palliative treatment but not for controlling cancer. Recently, alpha-emitting radionuclides have gained importance for their potential application to targeted cancer therapy (1,2) because of their short range and high Relative Biological Effectiveness. -

Spent Nuclear Fuel Analyses Based on In-Core Fuel Management Calculations
PHYSOR 2004 -The Physics of Fuel Cycles and Advanced Nuclear Systems: Global Developments Chicago, Illinois, April 25-29, 2004, on CD-ROM, American Nuclear Society, Lagrange Park, IL. (2004) Spent Nuclear Fuel Analyses based on In-Core Fuel Management Calculations Sigurd Børresen Studsvik Scandpower AS, P.O.15, NO-2027 Kjeller, Norway E-mail: [email protected] A method for performing spent nuclear fuel (SNF) analyses using in-core fuel management (ICFM) calculations has been developed and validated as described in this paper. Parameters such as decay heat, neutron source rates and photon spectra of discharged LWR fuel assemblies are calculated on the basis of ICFM core tracking data. This approach offers highly automated and accurate SNF analyses and eliminates the need for special fuel irradiation calculations to obtain the radiation source terms. This new SNF method has been validated against LWR spent fuel isotopic measurements, by comparisons with ORIGEN-ARP calculations and comparisons with a decay heat standard. A further validation effort against experimental decay heat data from a Swedish fuel storage facility is underway. KEYWORDS: Spent nuclear fuel, radiation source terms, decay heat, in-core fuel management. 1. Introduction Knowing the isotopic contents in the fuel at the time of discharge from the reactor is a prerequisite for back-end fuel cycle calculations with applications to storage, transportation and disposal of spent fuel. Solving the isotopic decay chains and using the summation method to calculate the radiation sources, these may be calculated at any time after discharge. The ENDF/B-VI and JEF-2.2 data libraries of CASMO-4 and HELIOS include a large number of actinides and fission products that are individually represented and tracked per fuel pin in ordinary ICFM fuel depletion calculations [1], [2]. -

Demonstration of a Rapid HPLC-ICPMS Direct Coupling Technique Using IDMS—Project Report: Part II
ORNL/TM-2017/697 Demonstration of a Rapid HPLC-ICPMS Direct Coupling Technique Using IDMS—Project Report: Part II Benjamin D. Roach David C. Glasgow Approved for public release. Emilie K. Fenske Distribution is unlimited. Ralph H. Ilgner Cole R. Hexel Joseph M. Giaquinto October 2017 DOCUMENT AVAILABILITY Reports produced after January 1, 1996, are generally available free via US Department of Energy (DOE) SciTech Connect. Website http://www.osti.gov/scitech/ Reports produced before January 1, 1996, may be purchased by members of the public from the following source: National Technical Information Service 5285 Port Royal Road Springfield, VA 22161 Telephone 703-605-6000 (1-800-553-6847) TDD 703-487-4639 Fax 703-605-6900 E-mail [email protected] Website http://classic.ntis.gov/ Reports are available to DOE employees, DOE contractors, Energy Technology Data Exchange representatives, and International Nuclear Information System representatives from the following source: Office of Scientific and Technical Information PO Box 62 Oak Ridge, TN 37831 Telephone 865-576-8401 Fax 865-576-5728 E-mail [email protected] Website http://www.osti.gov/contact.html This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof.