Neodymium, Samarium and Europium Capture Cross-Section Adjustments Based on Ebr-Ii Integral Measurements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Important Role of Dysprosium in Modern Permanent Magnets
The Important Role of Dysprosium in Modern Permanent Magnets Introduction Dysprosium is one of a group of elements called the Rare Earths. Rare earth elements consist of the Lanthanide series of 15 elements plus yttrium and scandium. Yttrium and scandium are included because of similar chemical behavior. The rare earths are divided into light and heavy based on atomic weight and the unique chemical and magnetic properties of each of these categories. Dysprosium (Figure 1) is considered a heavy rare earth element (HREE). One of the more important uses for dysprosium is in neodymium‐iron‐ boron (Neo) permanent magnets to improve the magnets’ resistance to demagnetization, and by extension, its high temperature performance. Neo magnets have become essential for a wide range of consumer, transportation, power generation, defense, aerospace, medical, industrial and other products. Along with terbium (Tb), Dysprosium (Dy) Figure 1: Dysprosium Metal(1) is also used in magnetostrictive devices, but by far the greater usage is in permanent magnets. The demand for Dy has been outstripping its supply. An effect of this continuing shortage is likely to be a slowing of the commercial rollout or a redesigning of a number of Clean Energy applications, including electric traction drives for vehicles and permanent magnet generators for wind turbines. The shortage and associated high prices are also upsetting the market for commercial and industrial motors and products made using them. Background Among the many figures of merit for permanent magnets two are of great importance regarding use of Dy. One key characteristic of a permanent magnet is its resistance to demagnetization, which is quantified by the value of Intrinsic Coercivity (HcJ or Hci). -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Some Structural Aspects of Neodymium Praseodymium Oxalate Single Crystals Grown in Hydro Silica Gels
Bull. Mater. Sci., Vol. 20, No. 1, February 1997, pp. 37-48. © Printed in India. Some structural aspects of neodymium praseodymium oxalate single crystals grown in hydro silica gels CYRIAC JOSEPH, M A ITTYACHEN and K S RAJU* School of Pure and Applied Phy~cs, Mahatma Gandhi University, Kottayam 686 031, India *Department of Crystallography and Biophysics, University of Madras, Guindy Campus, Madras 600 025, India MS received 25 September 1996; revised 14 November 1996 Abstract. The mixed crystals of neodymium praseodymium oxaiate are grown by the diffusion of a mixture of aqueous solutions of neodymium nitrate and praseodymium nitrate (as an upper reactant) into the set gel embedded with oxalic acid. By varying the concentration (by volume) of rare earth nitrates in the upper reactant, the incorporation of Nd and Pr in the mixed crystals has been studied. Tabular crystals with the well defined hexagonal basal planes are observed in the mixed crystals of varying concentrations. X-ray diffraction patterns of these powdered samples reveal that these mixed crystals are 'isostructural', while IR and FTIR spectra establish the presence of oxalate groups. TGA and DSC analyses show the correctness of the chemical formula for the mixed crystals, by the release of water molecules (endothermic) and of CO and CO2 (exothermic), with the rare earth oxides as the stable residue. X-ray fluorescence (XRF) and energy dispersive X-ray analyses (EDAX) establish the presence of heavy rare earth elements qualitatively and to a good extent quantitatively. X-ray photo- electron spectroscopic (XPS) studies confirm the presence of rare earth elements (Nd and Pr) as their respective oxides. -

Status of Fission Yield Measurements
nij»wiiM>iT»i»n'i»wwr I '• • «• PORTIOHS OF THIS &EPOTT ARE till •{•1& * i STATUS OF FI5.SIOM YIELD MEASUREHEMTS - i William J. Maeck Exxon Nuclear Idaho Company, Inc.* Idaho National Engineering Laboratory P.O. Box 2300 Idaho Fails, Idaho 83401, USA NOTICE . vtrpiird at Mt t*W. nm my < at i!tt$lte<K of anunwi my I*gal ! •prfn-nu Urn in uu *nuld noi | in(iuif.f privately 'IUWII n Prepared For Third ASTM-EURATOM Symposium On Reactor Dosimetry To be held at Ispra, Italy October 1-5, 1979 rUnder U^S... Department of Energy Idaho Operations Office Contract £- /L- - AC 07- 7c!XV>o 1. INTRODUCTION Fission yield data, for all neutron energies, play an important role in f many of the disiplines (fuel burnup, /ission rate measurements, dosimetry, reactor physics, damage studies, post irradiation fuel analysis, etc-) being discussed at this symposium. This paper reviews and presents a brief discussion i of the current status of fission yield measurement programs being conducted in the major laboratories throughout the world, and when possible, to—identify future activities. The contents of this paper are based on tv/o sources: 1) a mailing by the author to various principal investigators furnishing a form which could be used to describe their most current work, and 2) reference to the most recent IAEA Nuclear Data Section document "Progress in Fission Product Nuclear Data" , which is a compilation of current measurement activities relative i to fission product nuclear data, • • , The~gGnera-l-orgunizat.ij?Jl_oI._t]ie,.p.aper_Js..by-_f.issioning. -

Gjftjoh KB 06Ctivifibs
A gjftjOH KB EffBl&JS %ASI 06CTIVIfIBS IdXnmi 0SCIEEEEIQ5 thesis subs&ttea by 3BfcCBKSJUffBA OTA. H* 3©*, (University of Petna). far the degree of sector up raxes era* bepertnent of Seturel Philosophy, University of 2diobur,^i» HAY, 1950. C OB IE fl IS, Page IRTFOIJtJCTIGN • 1 SECT I OK I The JSatural Beta Activity of JBeodymium 3 "Results . » • • . 9 The effect of 3 elf-Ahsorption on the Estimate of Half life • • IS The Uncertainty in the Estimate of the Maximum Energy of f> -Particles • 16 The Isotoplo Assignment of the fieodymlum p -Activity * • 17 SECTIOB II Introduction * * « • . 30 Pleochroic Haloes . * . • 30 The Search for <k -Particles of Range about 2 cm amongst medium heavy Elements, • • . • 39 studies of Reodymium Activity with a Proportional Counter. • • 42 Experimental Results 47 Studies of -Particles from lecdymium in the Buclear Emulsion . 56 Experimental Results with nuclear w Emulsion. ♦ . • 59 Discussion ..... 63 CONTENTS (Oontd. ) Page SECTION II fContd*) ^-Activity araon^- the Ear© Earth Elements « • * * 69 isotypic Assignment • « « 76 SECTION III Studies In the Peaiosctivity of lanthanum 80 Experimental Arrangement 82 Experimental Results , ♦ * 82 Acknowledgments . , # 87 References , , • 89 £vvtsv3 efc • ^ ^<ry^c Uo-jkhs i^rc cry^jQX L (tJLc^J-Jt^ • f~ Hu = lii7 -s tlf.r' >• na. <°h 'Axa-oL AfT. ) 7«* -- 7-i2 . U- » 3,.o ^ A^L ,°/c> '«f «f- Mf J iX <vr "Vw\ <51 «v\ '. ^ ^ •=. XL. "U<* d ^NJ(, $ I v '<f«* ^■5^ ^V-0^ >5 IfO . t > . Is-' ^3 Jxwj flL «^u» ^w\jl"\ icLtxv» ^ V" twxxL-/ ^ - &\t-a-c-A*J<-A- f&Mx ~J *aaJI mU O-SV ca^U-t TLc y <~/^e)x*~* - 1 » 4 * k .31 . -

Interactions of Lanthanides and Liquid Alkali Metals for “Liquid-Like” Lanthanide Transport in U-Zr Fuel
Interactions of Lanthanides and Liquid Alkali Metals for “Liquid-Like” Lanthanide Transport in U-Zr Fuel THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Jeremy Payton Isler Graduate Program in Nuclear Engineering The Ohio State University 2017 Master's Examination Committee: Dr. Jinsuo Zhang, Advisor Dr. Marat Khafizov, Co-Advisor Copyrighted by Jeremy Payton Isler 2017 Abstract One of the major limitations in achieving increased burnup in metallic U-Zr nuclear fuel is Fuel-Cladding Chemical Interaction (FCCI). The migration of the fission product lanthanides from within the fuel to the peripheral is one of the major contributors to FCCI. A “liquid-like” transport mechanism proposes the lanthanide transport is aided by liquid cesium and liquid sodium in the pores of the fuel and at the fuel peripheral. The purpose of this thesis was to provide additional experimental evidence towards this proposed mechanism. This thesis investigated the interaction between the lanthanides and cesium and sodium, with a focus on the solubility of the lanthanides in these liquid alkali metals. First, a prediction of the solubility was calculated using the Miedema model. This prediction was then compared to the inversion crucible solubility experiment performed in this thesis. The solubility experiment studied the temperature and liquid-composition dependence of the lanthanides in liquid sodium, cesium and sodium-cesium mixtures. In addition, the solubility in mixtures shows the various alkali metals concentration effect on the solubility. With the results of differential scanning calorimetry (DSC) experiments, updated phase diagrams for sodium-neodymium and cesium-neodymium were obtained from the experimental data in this thesis. -

The Role of Cobalt in Neodymium Iron
TECHNotes The Role of Cobalt in NdFeB Permanent Magnets Neodymium iron boron (“Neo”) permanent magnets, discovered in 1980 and commercialized by 1984, remain the strongest magnets known to man – at least near room temperature. All magnets experience a change in properties as a function of temperature. As the Neo magnet temperature increases, both intrinsic coercivity, HcJ, and magnetic field strength, Br, decrease. Over a limited temperature range, these changes are non-destructive and reversible and are quantified by what are called Reversible Temperature Coefficients of Induction, (Br), and Intrinsic Coercivity (HcJ). These coefficients are the average rate of change between temperature limits. The changes are non-linear so it is necessary to state the temperature range over which the coefficients are calculated. To permit Neo magnets to operate at elevated temperatures, two composition changes are introduced. Figure 1. Magnetic field strength of iron, cobalt and nickel as a 1. The substitution of the heavy rare earth elements function of temperature. [1] (HREEs) dysprosium and/or terbium for a portion of the neodymium increases the anisotropy field. This provides higher room temperature intrinsic coercivity and reduces the rate at which coercivity drops as temperature increases – smaller reversible temperature coefficient of (intrinsic) coercivity. 2. Substitution of cobalt for a portion of iron raises the magnet Curie temperature, Tc, and reduces the rate at which magnetic field strength, B, changes as a function of temperature. This TECHNote focuses on the effects of cobalt on magnet Curie temperature, residual induction, intrinsic coercivity and corrosion resistance. Curie Temperature Figure 2. Magnetization versus magnetic field strength for Both ferro- and ferrimagnetic materials exhibit a NdFeB magnets with three levels of cobalt. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Effect of the Addition of Low Rare Earth Elements (Lanthanum, Neodymium, Cerium) on the Biodegradation and Biocompatibility of Magnesium
Acta Biomaterialia 11 (2015) 554–562 Contents lists available at ScienceDirect Acta Biomaterialia journal homepage: www.elsevier.com/locate/actabiomat Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium Elmar Willbold a,b,1, Xuenan Gu c,d,1, Devon Albert a,b,e, Katharina Kalla a,b, Katharina Bobe a,b, Maria Brauneis a,b, Carla Janning a,b, Jens Nellesen f, Wolfgang Czayka f, Wolfgang Tillmann f, ⇑ ⇑ Yufeng Zheng c, , Frank Witte a,b,2, a Laboratory for Biomechanics and Biomaterials, Department of Orthopaedic Surgery, Hannover Medical School, Anna-von-Borries-Straße 1-7, 30625 Hannover, Germany b CrossBIT, Center for Biocompatibility and Implant-Immunology, Department of Orthopaedic Surgery, Hannover Medical School, Feodor-Lynen-Straße 31, 30625 Hannover, Germany c Department of Materials Science and Engineering, College of Engineering, Peking University, No. 5 Yi-He-Yuan Road, Hai-Dian District, Beijing 100871, China d Key Laboratory for Biomechanics and Mechanobiology of Ministry of Education, School of Biological Science and Medical Engineering, Beihang University, Beijing 100191, China e Swanson School of Engineering, Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA f Institute of Materials Engineering, Technische Universität Dortmund, Leonhard-Euler-Straße 2, 44227 Dortmund, Germany article info abstract Article history: Rare earth elements are promising alloying element candidates for magnesium alloys used as biodegrad- Received 7 May 2014 able devices in biomedical applications. Rare earth elements have significant effects on the high temper- Received in revised form 15 September ature strength as well as the creep resistance of alloys and they improve magnesium corrosion resistance. -
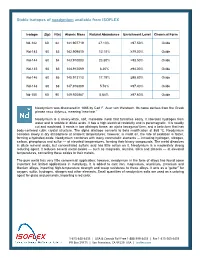
Stable Isotopes of Neodymium Available from ISOFLEX
Stable isotopes of neodymium available from ISOFLEX Isotope Z(p) N(n) Atomic Mass Natural Abundance Enrichment Level Chemical Form Nd-142 60 82 141.907719 27.13% >97.50% Oxide Nd-143 60 83 142.909810 12.18% ≥79.00% Oxide Nd-144 60 84 143.910083 23.80% >98.50% Oxide Nd-145 60 85 144.912569 8.30% ≥94.00% Oxide Nd-146 60 86 145.913113 17.19% ≥98.80% Oxide Nd-148 60 88 147.916889 5.76% ≥97.40% Oxide Nd-150 60 90 149.920887 5.64% ≥97.60% Oxide Neodymium was discovered in 1885 by Carl F. Auer von Welsbach. Its name derives from the Greek phrase neos didymos, meaning “new twin.” Neodymium is a silvery-white, soft, malleable metal that tarnishes easily. It liberates hydrogen from water and is soluble in dilute acids. It has a high electrical resistivity and is paramagnetic. It is readily cut and machined. It exists in two allotropic forms: an alpha hexagonal form, and a beta form that has body-centered cubic crystal structure. The alpha allotrope converts to beta modification at 868 ºC. Neodymium corrodes slowly in dry atmosphere at ambient temperatures; however, in moist air, the rate of oxidation is faster, forming a hydrated oxide. Neodymium combines with many nonmetallic elements — including hydrogen, nitrogen, carbon, phosphorus and sulfur — at elevated temperatures, forming their binary compounds. The metal dissolves in dilute mineral acids, but concentrated sulfuric acid has little action on it. Neodymium is a moderately strong reducing agent. It reduces several metal oxides — such as magnesia, alumina, silica and zirconia — at elevated temperatures, converting these oxides to their metals. -

Influence of Size of Neodymium:Yttrium-Aluminium-Garnet Laser Posterior Capsulotomy on Visual Function
Eye (2010) 24, 101–106 & 2010 Macmillan Publishers Limited All rights reserved 0950-222X/10 $32.00 www.nature.com/eye 1 1 2 Influence of size of K Hayashi , F Nakao and H Hayashi CLINICAL STUDY neodymium:yttrium- aluminium-garnet laser posterior capsulotomy on visual function Abstract Introduction Purpose The aim of this study was to It has been shown that posterior capsule examine the influence that the size of a opacification (PCO) following cataract surgery neodymium:yttrium-aluminium-garnet disturbs various visual functions, including (Nd:YAG) laser capsulotomy performed visual acuity (VA), contrast sensitivity and glare for posterior capsule opacification (PCO) disability, although controversy still remains as has on visual acuity (VA), and on contrast to which function is impaired most prominently VA and that in the presence of glare due to PCO.1–9 However, we believe that the (glare VA). impairment of visual functions is related to the Methods A total of 41 consecutive eyes with degree of PCO. In eyes with dense PCO that PCO first underwent Nd:YAG laser require neodymium:yttrium-aluminium-garnet capsulotomy of smaller than pupillary size, (Nd:YAG) laser posterior capsulotomy, as all after which the capsulotomy was secondarily visual functions are impaired, VA is associated 1Hayashi Eye Hospital, enlarged, 2 weeks later, to greater than most strongly with the degree of PCO.7 When Fukuoka, Japan pupillary size. Best-corrected VA, and contrast the PCO is slight, however, contrast sensitivity 2 VA and glare VA under photopic and mesopic or glare disability deteriorates with no marked Department of Ophthalmology, School of conditions were measured after the small and loss of VA.8 Medicine, Fukuoka large capsulotomies were made. -

Developing a High Grade Neodymium & Praseodymium Mine
Developing a High Grade Neodymium & Praseodymium Mine HASTINGS Investor Presentation Technology Metals Limited July 2018 All currency amounts are in A$ unless stated otherwise. Disclaimer This presentation has been prepared by Hastings Technology Metals Limited (“Company”). It does not purport to contain all the information that a prospective investor may require in connection with any potential investment in the Company. You should not treat the contents of this presentation, or any information provided in connection with it, as financial advice, financial product advice or advice relating to legal, taxation or investment matters. This presentation is provided expressly on the basis that you will carry out your own independent inquiries into the matters contained in the presentation and make your own independent decisions about the affairs, financial position or prospects of the Company. The Company reserves the right to update, amend or supplement the information at any time in its absolute discretion (without incurring any obligation to do so). Neither the Company, nor its related bodies corporate, officers, their advisers, agents and employees accept any responsibility or liability to any person or entity as to the accuracy, completeness or reasonableness of the information, statements, opinions or matters (express or implied) arising out of, contained in or derived from this presentation or provided in connection with it, or any omission from this presentation, nor as to the attainability of any estimates, forecasts or projections set out in this presentation. Pursuant to the general law (whether for negligence, under statute or otherwise), or any Australian legislation or any other jurisdiction. Any such responsibility or liability is, to the maximum extent permitted by law, expressly disclaimed and excluded.