Production and Separation of Terbium-149 and Terbium-152 For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Important Role of Dysprosium in Modern Permanent Magnets
The Important Role of Dysprosium in Modern Permanent Magnets Introduction Dysprosium is one of a group of elements called the Rare Earths. Rare earth elements consist of the Lanthanide series of 15 elements plus yttrium and scandium. Yttrium and scandium are included because of similar chemical behavior. The rare earths are divided into light and heavy based on atomic weight and the unique chemical and magnetic properties of each of these categories. Dysprosium (Figure 1) is considered a heavy rare earth element (HREE). One of the more important uses for dysprosium is in neodymium‐iron‐ boron (Neo) permanent magnets to improve the magnets’ resistance to demagnetization, and by extension, its high temperature performance. Neo magnets have become essential for a wide range of consumer, transportation, power generation, defense, aerospace, medical, industrial and other products. Along with terbium (Tb), Dysprosium (Dy) Figure 1: Dysprosium Metal(1) is also used in magnetostrictive devices, but by far the greater usage is in permanent magnets. The demand for Dy has been outstripping its supply. An effect of this continuing shortage is likely to be a slowing of the commercial rollout or a redesigning of a number of Clean Energy applications, including electric traction drives for vehicles and permanent magnet generators for wind turbines. The shortage and associated high prices are also upsetting the market for commercial and industrial motors and products made using them. Background Among the many figures of merit for permanent magnets two are of great importance regarding use of Dy. One key characteristic of a permanent magnet is its resistance to demagnetization, which is quantified by the value of Intrinsic Coercivity (HcJ or Hci). -

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Rare Earth Elements in National Defense: Background, Oversight Issues, and Options for Congress
Rare Earth Elements in National Defense: Background, Oversight Issues, and Options for Congress Valerie Bailey Grasso Specialist in Defense Acquisition December 23, 2013 Congressional Research Service 7-5700 www.crs.gov R41744 Rare Earth Elements in National Defense Summary Some Members of Congress have expressed concern over U.S. acquisition of rare earth materials composed of rare earth elements (REE) used in various components of defense weapon systems. Rare earth elements consist of 17 elements on the periodic table, including 15 elements beginning with atomic number 57 (lanthanum) and extending through number 71 (lutetium), as well as two other elements having similar properties (yttrium and scandium). These are referred to as “rare” because although relatively abundant in total quantity, they appear in low concentrations in the earth’s crust and extraction and processing is both difficult and costly. From the 1960s to the 1980s, the United States was the leader in global rare earth production. Since then, production has shifted almost entirely to China, in part due to lower labor costs and lower environmental standards. Some estimates are that China now produces about 90- 95% of the world’s rare earth oxides and is the majority producer of the world’s two strongest magnets, samarium cobalt (SmCo) and neodymium iron boron (NeFeB) permanent, rare earth magnets. In the United States, Molycorp, a Mountain Pass, CA mining company, recently announced the purchase of Neo Material Technologies. Neo Material Technologies makes specialty materials from rare earths at factories based in China and Thailand. Molycorp also announced the start of its new heavy rare earth production facilities, Project Phoenix, which will process rare earth oxides from ore mined from the Mountain Pass facilities. -

Status of Fission Yield Measurements
nij»wiiM>iT»i»n'i»wwr I '• • «• PORTIOHS OF THIS &EPOTT ARE till •{•1& * i STATUS OF FI5.SIOM YIELD MEASUREHEMTS - i William J. Maeck Exxon Nuclear Idaho Company, Inc.* Idaho National Engineering Laboratory P.O. Box 2300 Idaho Fails, Idaho 83401, USA NOTICE . vtrpiird at Mt t*W. nm my < at i!tt$lte<K of anunwi my I*gal ! •prfn-nu Urn in uu *nuld noi | in(iuif.f privately 'IUWII n Prepared For Third ASTM-EURATOM Symposium On Reactor Dosimetry To be held at Ispra, Italy October 1-5, 1979 rUnder U^S... Department of Energy Idaho Operations Office Contract £- /L- - AC 07- 7c!XV>o 1. INTRODUCTION Fission yield data, for all neutron energies, play an important role in f many of the disiplines (fuel burnup, /ission rate measurements, dosimetry, reactor physics, damage studies, post irradiation fuel analysis, etc-) being discussed at this symposium. This paper reviews and presents a brief discussion i of the current status of fission yield measurement programs being conducted in the major laboratories throughout the world, and when possible, to—identify future activities. The contents of this paper are based on tv/o sources: 1) a mailing by the author to various principal investigators furnishing a form which could be used to describe their most current work, and 2) reference to the most recent IAEA Nuclear Data Section document "Progress in Fission Product Nuclear Data" , which is a compilation of current measurement activities relative i to fission product nuclear data, • • , The~gGnera-l-orgunizat.ij?Jl_oI._t]ie,.p.aper_Js..by-_f.issioning. -

Gjftjoh KB 06Ctivifibs
A gjftjOH KB EffBl&JS %ASI 06CTIVIfIBS IdXnmi 0SCIEEEEIQ5 thesis subs&ttea by 3BfcCBKSJUffBA OTA. H* 3©*, (University of Petna). far the degree of sector up raxes era* bepertnent of Seturel Philosophy, University of 2diobur,^i» HAY, 1950. C OB IE fl IS, Page IRTFOIJtJCTIGN • 1 SECT I OK I The JSatural Beta Activity of JBeodymium 3 "Results . » • • . 9 The effect of 3 elf-Ahsorption on the Estimate of Half life • • IS The Uncertainty in the Estimate of the Maximum Energy of f> -Particles • 16 The Isotoplo Assignment of the fieodymlum p -Activity * • 17 SECTIOB II Introduction * * « • . 30 Pleochroic Haloes . * . • 30 The Search for <k -Particles of Range about 2 cm amongst medium heavy Elements, • • . • 39 studies of Reodymium Activity with a Proportional Counter. • • 42 Experimental Results 47 Studies of -Particles from lecdymium in the Buclear Emulsion . 56 Experimental Results with nuclear w Emulsion. ♦ . • 59 Discussion ..... 63 CONTENTS (Oontd. ) Page SECTION II fContd*) ^-Activity araon^- the Ear© Earth Elements « • * * 69 isotypic Assignment • « « 76 SECTION III Studies In the Peaiosctivity of lanthanum 80 Experimental Arrangement 82 Experimental Results , ♦ * 82 Acknowledgments . , # 87 References , , • 89 £vvtsv3 efc • ^ ^<ry^c Uo-jkhs i^rc cry^jQX L (tJLc^J-Jt^ • f~ Hu = lii7 -s tlf.r' >• na. <°h 'Axa-oL AfT. ) 7«* -- 7-i2 . U- » 3,.o ^ A^L ,°/c> '«f «f- Mf J iX <vr "Vw\ <51 «v\ '. ^ ^ •=. XL. "U<* d ^NJ(, $ I v '<f«* ^■5^ ^V-0^ >5 IfO . t > . Is-' ^3 Jxwj flL «^u» ^w\jl"\ icLtxv» ^ V" twxxL-/ ^ - &\t-a-c-A*J<-A- f&Mx ~J *aaJI mU O-SV ca^U-t TLc y <~/^e)x*~* - 1 » 4 * k .31 . -

Terbium Nanoparticle Biofunctionalization for Extracellular Biosensing Vjona Çifliku
Terbium nanoparticle biofunctionalization for extracellular biosensing Vjona Çifliku To cite this version: Vjona Çifliku. Terbium nanoparticle biofunctionalization for extracellular biosensing. Inorganic chem- istry. Université Paris-Saclay, 2020. English. NNT : 2020UPASS075. tel-02938737 HAL Id: tel-02938737 https://tel.archives-ouvertes.fr/tel-02938737 Submitted on 15 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Terbium nanoparticle biofunctionalization for extracellular biosensing Thèse de doctorat de l'université Paris-Saclay École doctorale n°575: Electrical, optical, bio: physics and engineering (EOBE) Spécialité de doctorat: Physique (Electronique et Optoélectronique, Nano et Microtechnologies) Unité de recherche : Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC), 91198, Gif-sur-Yvette, France Référent : Faculté des sciences Thèse présentée et soutenue à Orsay, le 9 Juin 2020, par Vjona Çifliku Composition du Jury Sandrine LACOMBE Présidente Professeure, Université Paris Saclay Alexandra -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Cluster of Study of Terbium's Journey from Mine to 21St Century
Volume 65, Issue 2, 2021 Journal of Scientific Research Institute of Science, Banaras Hindu University, Varanasi, India. Cluster of Study of Terbium’s Journey From Mine To 21st Century Nishigandha Khairnar Jnan Vikas Mandal’s Degree College. [email protected] Abstract: An element found in the Ytterby mine, with 65 atomic quite ironic the abundance is 20 times that of silver so it's not number, nestled between its brother's gadolinium and dysprosium that rare, its magnetic property is used in nanomaterials, due to in the lanthanide series is a unique element. Efficient magneto triction, shown by alloy of terbium. Teflon-D an its super paramagnetic form, therefore its intensely researched as alloy of terbium, when magnetic field passed through has a unique some elements lose their magnetic and chemical properties with character of, increasing and decreasing in its size. Is highly desired decrease in its size unlike terbium. For example, cuprite in the fields of nanotubes and materials. Herein, a new alloy with its nanomaterial of terbium complexes is used in aircrafts or engine, unique property is anticipated to enhance the world of for detection of damage in machine parts or as a replacement for microfabrication research. Showing phosphorescence and its those parts. Scientist come up with various technique to make elaborate uses, would be used to stain biological cells, without any much more efficient nickel terbium complex. In the present biological activity, would cut the exposure to radioactive material. paper well learn more about its complexes and alloys. Its property is in demand for low-energy lightbulbs and mercury Terbium complexes found in late 1900’s with nickel, copper and lamps. -
BLM Alaska Rare Earth Elements Infographic
MINERALS ARE CRITICAL TO A RENEWABLE FUTURE RARE EARTH ELEMENTS Alaska may hold what the nation needs to innovate for tomorrow Rare Earth Elements (REE) are a group of 17 metals that Periodic Table cannot be separated into simpler substances by chemical means, which is why they appear together in the periodic table. They occur abundantly on earth but are rarely found in concentrations high enough for economical extraction; thus their REE nickname. They are soft, malleable, and ductile and usually reactive, especially at elevated temperatures or when finely divided. The rare earth elements' unique properties are 15 within the chemical group called LANTHANIDES used in a wide variety of applications. plus SCANDIUM and YTTRIUM RARE EARTH ELEMENTS La Ce Pr No Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Sc Y Occurrences in Alaska Even green tech generates waste UTQIAĠVIK With fewer than 5% of lithium-ion batteries being recycled worldwide in 2019, for example, we can PRUDHOE BAY all reduce e-waste by recycling the REE already mined. NOME FAIRBANKS ANCHORAGE JUNEAU SITKA Alaska REEs KETCHIKAN Bokan Mountain Property, La lanthanum Ho holmium 37 miles southwest of Ketchikan Ce cerium Er erbium on Prince of Wales Island, is Alaska’s Gd gadolinium Yb ytterbium most significant REE prospect. Dy dysprosium Y yttrium Map is for graphical purposes only 2011 Data Rare Earth Minerals in Mobile Devices Vibrator Dysprosium Circuit board electronics Neodymium Dysprosium Praseodymium Gadolinum Terbium Lanthanum Neodymium Praseodymium Color Screen Dysprosium Europium Lanthanum Neodymium Praseodymium Terbium Yttrium Glass polishing Cerium Lanthanum Praseodymium Speaker Dysprosium Neodymium Praseodymium Terbium References: https://dggs.alaska.gov https://www.usgs.gov For more information, visit https://www.blm.gov/alaska/minerals https://www.inl.gov https://www.rare-earths.com. -
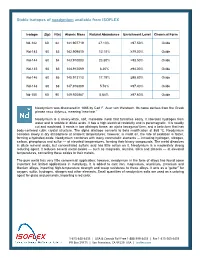
Stable Isotopes of Neodymium Available from ISOFLEX
Stable isotopes of neodymium available from ISOFLEX Isotope Z(p) N(n) Atomic Mass Natural Abundance Enrichment Level Chemical Form Nd-142 60 82 141.907719 27.13% >97.50% Oxide Nd-143 60 83 142.909810 12.18% ≥79.00% Oxide Nd-144 60 84 143.910083 23.80% >98.50% Oxide Nd-145 60 85 144.912569 8.30% ≥94.00% Oxide Nd-146 60 86 145.913113 17.19% ≥98.80% Oxide Nd-148 60 88 147.916889 5.76% ≥97.40% Oxide Nd-150 60 90 149.920887 5.64% ≥97.60% Oxide Neodymium was discovered in 1885 by Carl F. Auer von Welsbach. Its name derives from the Greek phrase neos didymos, meaning “new twin.” Neodymium is a silvery-white, soft, malleable metal that tarnishes easily. It liberates hydrogen from water and is soluble in dilute acids. It has a high electrical resistivity and is paramagnetic. It is readily cut and machined. It exists in two allotropic forms: an alpha hexagonal form, and a beta form that has body-centered cubic crystal structure. The alpha allotrope converts to beta modification at 868 ºC. Neodymium corrodes slowly in dry atmosphere at ambient temperatures; however, in moist air, the rate of oxidation is faster, forming a hydrated oxide. Neodymium combines with many nonmetallic elements — including hydrogen, nitrogen, carbon, phosphorus and sulfur — at elevated temperatures, forming their binary compounds. The metal dissolves in dilute mineral acids, but concentrated sulfuric acid has little action on it. Neodymium is a moderately strong reducing agent. It reduces several metal oxides — such as magnesia, alumina, silica and zirconia — at elevated temperatures, converting these oxides to their metals.