Effects of Land-Use Intensity in Grasslands on Diversity, Life-History Traits and Multitrophic Interactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fauna Lepidopterologica Volgo-Uralensis" 150 Years Later: Changes and Additions
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2000) 31 (1/2):327-367< Würzburg, ISSN 0171-0079 "Fauna lepidopterologica Volgo-Uralensis" 150 years later: changes and additions. Part 5. Noctuidae (Insecto, Lepidoptera) by Vasily V. A n ik in , Sergey A. Sachkov , Va d im V. Z o lo t u h in & A n drey V. Sv ir id o v received 24.II.2000 Summary: 630 species of the Noctuidae are listed for the modern Volgo-Ural fauna. 2 species [Mesapamea hedeni Graeser and Amphidrina amurensis Staudinger ) are noted from Europe for the first time and one more— Nycteola siculana Fuchs —from Russia. 3 species ( Catocala optata Godart , Helicoverpa obsoleta Fabricius , Pseudohadena minuta Pungeler ) are deleted from the list. Supposedly they were either erroneously determinated or incorrect noted from the region under consideration since Eversmann 's work. 289 species are recorded from the re gion in addition to Eversmann 's list. This paper is the fifth in a series of publications1 dealing with the composition of the pres ent-day fauna of noctuid-moths in the Middle Volga and the south-western Cisurals. This re gion comprises the administrative divisions of the Astrakhan, Volgograd, Saratov, Samara, Uljanovsk, Orenburg, Uralsk and Atyraus (= Gurjev) Districts, together with Tataria and Bash kiria. As was accepted in the first part of this series, only material reliably labelled, and cover ing the last 20 years was used for this study. The main collections are those of the authors: V. A n i k i n (Saratov and Volgograd Districts), S. -

Новые Сведения По Фауне Пядениц (Lepidoptera, Geometridae) Якутии
Труды Русского энтомологического общества. С.‐Петербург, 2011. Т. 82: 57–66. Proceedings of the Russian Entomological Society. St. Petersburg, 2011. Vol. 82: 57–66. Новые сведения по фауне пядениц (Lepidoptera, Geometridae) Якутии. I А.П. Бурнашева1, Е.А. Беляев2 New data on the fauna of geometrid moths (Lepidoptera, Geometridae) of Yakutia. I A.P. Burnasheva1, E.A. Beljaev2 1Институт биологических проблем криолитозоны СО РАН, пр. Ленина 41, Якутск 677980, Россия. Institute for Biological Problems of Cryolithozone SB RAS, Lenin av., 41, Yakutsk 677980, Russia. E-mail: [email protected] 2Биолого-почвенный институт ДВО РАН, пр. 100 лет Владивостоку 159, Владивосток 690022, Россия. Institute for Biology and Soil Sciences FEB RAS, 100 let Vladivostoku av., 159, Vladivostok 690022, Russia. Е-mail: [email protected] Резюме. В статье рассмотрены 23 вида пядениц , из которых 10 видов (Macaria signaria Hbn., Tha- lera fimbrialis Scop., Idaea biselata Hufn., Timandra griseata W. Peters., Xanthorhoe designata Hufn., Plemyria rubiginata Den. et Schiff., Perizoma alchemillata L., Eupithecia subfuscata Haw., E. succen- turiata L., Pterapherapteryx sexalata Retz.) являются новыми для территории Якутии, а 13 – малоиз- вестными, по которым получены новые материалы. Указания для Якутии Rhopalognophos glauci- naria Hbn. и Idaea serpentata Hufn., вероятно, являются ошибочными. По каждому виду приводят- ся сведения об исследованном материале, распространении и кормовых растениях гусениц. Ключевые слова. Lepidoptera, Geometridae, новые находки, редкие виды, Якутия. Abstract. Twenty three species of geometrid moths are discussed in the paper. Ten species of this moths are recorded from Yakutia for the first time (Macaria signaria Hbn., Thalera fimbrialis Scop., Idaea biselata Hufn., Timandra griseata W.Peters., Xanthorhoe designata Hufn., Plemyria rubiginata Den. -

Climate Change and Conservation of Orophilous Moths at the Southern Boundary of Their Range (Lepidoptera: Macroheterocera)
Eur. J. Entomol. 106: 231–239, 2009 http://www.eje.cz/scripts/viewabstract.php?abstract=1447 ISSN 1210-5759 (print), 1802-8829 (online) On top of a Mediterranean Massif: Climate change and conservation of orophilous moths at the southern boundary of their range (Lepidoptera: Macroheterocera) STEFANO SCALERCIO CRA Centro di Ricerca per l’Olivicoltura e l’Industria Olearia, Contrada Li Rocchi-Vermicelli, I-87036 Rende, Italy; e-mail: [email protected] Key words. Biogeographic relict, extinction risk, global warming, species richness, sub-alpine prairies Abstract. During the last few decades the tree line has shifted upward on Mediterranean mountains. This has resulted in a decrease in the area of the sub-alpine prairie habitat and an increase in the threat to strictly orophilous moths that occur there. This also occurred on the Pollino Massif due to the increase in temperature and decrease in rainfall in Southern Italy. We found that a number of moths present in the alpine prairie at 2000 m appear to be absent from similar habitats at 1500–1700 m. Some of these species are thought to be at the lower latitude margin of their range. Among them, Pareulype berberata and Entephria flavicinctata are esti- mated to be the most threatened because their populations are isolated and seem to be small in size. The tops of these mountains are inhabited by specialized moth communities, which are strikingly different from those at lower altitudes on the same massif further south. The majority of the species recorded in the sub-alpine prairies studied occur most frequently and abundantly in the core area of the Pollino Massif. -

Variation in the Habitat Requirements of Pollinating Insects in Semi-Natural Meadows
VARIATION IN THE HABITAT REQUIREMENTS OF POLLINATING INSECTS IN SEMI-NATURAL MEADOWS TOLMELDAJATE ELUPAIGANÕUDLUSTE MITMEKESISUS POOLLOODUSLIKES KOOSLUSTES AVE LIIVAMÄGI A Thesis for applying for the degree of Doctor of Philosophy in Environmental protection Väitekiri filosoofiadoktori kraadi taotlemiseks keskkonnakaitse erialal Tartu 2014 EESTI MAAÜLIKOOL ESTONIAN UNIVERSITY OF LIFE SCIENCES VARIATION IN THE HABITAT REQUIREMENTS OF POLLINATING INSECTS IN SEMI-NATURAL MEADOWS TOLMELDAJATE ELUPAIGANÕUDLUSTE MITMEKESISUS POOLLOODUSLIKES KOOSLUSTES AVE LIIVAMÄGI A Thesis for applying for the degree of Doctor of Philosophy in Environmental protection Väitekiri fi losoofi adoktori kraadi taotlemiseks keskkonnakaitse erialal Tartu 2014 Institute of Agricultural and Environmental Sciences Estonian University of Life Sciences According to the verdict No 172 of March 27, 2014, the Doctoral Committee of Agricultural and Natural Sciences of the Estonian University of Life Sciences has accepted the thesis for the defence of the degree of Doctor of Philosophy in Environmental Protection. Opponent: Prof. Miska Luoto Department of Geosciences and Geography University of Helsinki Helsinki, Finland Supervisor: Prof. Valdo Kuusemets Institute of Agricultural and Environmental Sciences Estonian University of Life Sciences Tartu, Estonia Defence of the thesis: Estonian University of Life Sciences, room 1A5, Kreutzwaldi 5, Tartu. May 9, 2014, at 14:00. The English language was edited by Elsevier language editors and the Estonian language by Kristi Liivamägi. Publication of this thesis is supported by the Estonian University of Life Sciences and by the Doctoral School of Earth Sciences and Ecology created under the auspices of European Social Fund. © Ave Liivamägi, 2014 ISBN 978-9949-536-25-2 (trükis) ISBN 978-9949-536-26-9 (pdf) CONTENTS LIST OF ORIGINAL PUBLICATIONS .................................................7 ABBREVIATIONS .......................................................................................9 1. -

Volume 59 – June 2017
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Newsletter of the Societas Europaea Lepidopterologica Jahr/Year: 2017 Band/Volume: 59 Autor(en)/Author(s): diverse Artikel/Article: Newsletter of the Societas Europaea Lepidopterologica 59 1 Volume 59 – June 2017 The Newsletter of the Societas Europaea Lepidopterologica A kind reminder: please pay your membership fee – Rappel pour les paiements – Zur Erinnerung: bitte zahlen Sie Ihren Mitgliedsbeitrag ......................................................2 2017 SEL General Meeting – Assemblée Générale – Generalversammlung ....................3–7 New Council members 2017 ..............................................................................................8 2017 SEL Council Report – Rapport du Conceil – Bericht des Vorstandes .....................9–13 New SEL committees: a call for collaboration! .................................................................13 2017 SEL Treasurer’s report – Rapport financier – Kassenbericht ...............................14–19 New Treasurer: handing over the documents – Nouveau Trésorier: passation des documents – Neuer Schatzmeister: Übergabe der Unterlagen ...................................21 Changes to the list of members – Änderungen zur Mitgliederliste – Changements à la liste des membres ...............................................................................................................22 SEL congress impressions ...........................................................................................23–28 -

Melitaea Britomartis ASSMANN, 1847 Am Nordwestlichen Rand Des Artareals (Lepidoptera, Nymphalidae)
Entomologische Nachrichten und Berichte, 59, 2015/1 33 T. MEINEKE & KERSTIN MENGE, Ebergötzen Weitere Funde von Melitaea britomartis ASSMANN, 1847 am nordwestlichen Rand des Artareals (Lepidoptera, Nymphalidae) Zusammenfassung Im Jahr 2014 gelangen im nordwestlichen Thüringen an sieben Örtlichkeiten Nachweise von Melitaea britomartis ASSMANN, 1847. Im Vergleich mit der laut Schrifttum bekannten Verbreitung sind die Funde neu. Sie liegen an der nordwestlichen Grenze des Artareals. M. britomartis trat an vier Flugplätzen gemeinsam mit M. aurelia NICKERL, 1850 auf, an einem außerdem zusammen mit M. athalia (ROTTEMBURG, 1775). Jede der drei Scheckenfalterarten variiert in ihren äußerlichen Merkmalen erheblich. Hinsichtlich Flügelgröße, Färbung der Palpen und Farbintensität des Submarginalbandes auf der Hinterflügelunterseite gibt es große Überschneidungsbereiche, die eine sichere Determination ausschließen. Hingegen ermöglicht die Heranziehung der Kopulationsorgane in beiden Geschlechtern eine problemlose Unterscheidung der drei Arten. Anhand eines skizzenhaften Rückblickes auf die Geschichte der taxonomischen wie faunistischen Wahrnehmung von M. britomartis wird der Versuch unternommen, die mitgeteilten Funde im Kontext der gegenwärtig bekannten Verbreitung zu bewerten. S u m m a r y Further records of Melitaea britomartis ASSMANN, 1847 from the northwestern edge of its range (Lepidoptera, Nymphalidae). - In 2014 Melitaea britomartis ASSMANN, 1847 was recorded at seven sites in northwest Thuringia. From literature, all of these records are new and lie at the northwestern border of the species’ range. At four sites, M. britomartis occurred together with M. aurelia NICKERL, 1850, at one site additionally with M. athalia (ROTTEMBURG, 1775). External characters vary considerably in each of these three Fritillary species. Overlap in wing size, pigmentation of palpi, and colour intensity of the submarginal band on the lower face of the hindwing excludes reliable identification. -

Contribution to the Knowledge of the Fauna of Bombyces, Sphinges And
driemaandelijks tijdschrift van de VLAAMSE VERENIGING VOOR ENTOMOLOGIE Afgiftekantoor 2170 Merksem 1 ISSN 0771-5277 Periode: oktober – november – december 2002 Erkenningsnr. P209674 Redactie: Dr. J–P. Borie (Compiègne, France), Dr. L. De Bruyn (Antwerpen), T. C. Garrevoet (Antwerpen), B. Goater (Chandlers Ford, England), Dr. K. Maes (Gent), Dr. K. Martens (Brussel), H. van Oorschot (Amsterdam), D. van der Poorten (Antwerpen), W. O. De Prins (Antwerpen). Redactie-adres: W. O. De Prins, Nieuwe Donk 50, B-2100 Antwerpen (Belgium). e-mail: [email protected]. Jaargang 30, nummer 4 1 december 2002 Contribution to the knowledge of the fauna of Bombyces, Sphinges and Noctuidae of the Southern Ural Mountains, with description of a new Dichagyris (Lepidoptera: Lasiocampidae, Endromidae, Saturniidae, Sphingidae, Notodontidae, Noctuidae, Pantheidae, Lymantriidae, Nolidae, Arctiidae) Kari Nupponen & Michael Fibiger [In co-operation with Vladimir Olschwang, Timo Nupponen, Jari Junnilainen, Matti Ahola and Jari- Pekka Kaitila] Abstract. The list, comprising 624 species in the families Lasiocampidae, Endromidae, Saturniidae, Sphingidae, Notodontidae, Noctuidae, Pantheidae, Lymantriidae, Nolidae and Arctiidae from the Southern Ural Mountains is presented. The material was collected during 1996–2001 in 10 different expeditions. Dichagyris lux Fibiger & K. Nupponen sp. n. is described. 17 species are reported for the first time from Europe: Clostera albosigma (Fitch, 1855), Xylomoia retinax Mikkola, 1998, Ecbolemia misella (Püngeler, 1907), Pseudohadena stenoptera Boursin, 1970, Hadula nupponenorum Hacker & Fibiger, 2002, Saragossa uralica Hacker & Fibiger, 2002, Conisania arida (Lederer, 1855), Polia malchani (Draudt, 1934), Polia vespertilio (Draudt, 1934), Polia altaica (Lederer, 1853), Mythimna opaca (Staudinger, 1899), Chersotis stridula (Hampson, 1903), Xestia wockei (Möschler, 1862), Euxoa dsheiron Brandt, 1938, Agrotis murinoides Poole, 1989, Agrotis sp. -

Land-Use Changes, Farm Management and the Decline of Butterflies Associated with Semi-Natural Grasslands in Southern Sweden
A peer-reviewed open-access journal Nature Conservation Land-use6: 31–48 (2013) changes, farm management and the decline of butterflies.... 31 doi: 10.3897/natureconservation.6.5205 APPLIED ECOLOGY http://www.pensoft.net/natureconservation Launched to accelerate biodiversity conservation Land-use changes, farm management and the decline of butterflies associated with semi-natural grasslands in southern Sweden Sven G. Nilsson1, Markus Franzén1,2, Lars B. Pettersson1,3 1 Biodiversity Unit, Department of Biology, Lund University, Ecology Building, SE-223 62 Lund, Sweden 2 UFZ Helmholtz Centre for Environmental Research, Department of Community Ecology, Theodor-Lieser- Straße 4, D-06120 Halle, Germany 3 Swedish Butterfly Monitoring Scheme, Lund University, Ecology Buil- ding, SE-223 62 Lund, Sweden Corresponding author: Lars B. Pettersson ([email protected]) Academic editor: L. Penev | Received 26 March 2013 | Accepted 30 October 2013 | Published 18 November 2013 Citation: Nilsson SG, Franzén M, Pettersson LB (2013) Land-use changes, farm management and the decline of butterflies associated with semi-natural grasslands in southern Sweden. Nature Conservation 18: 31–48. doi: 10.3897/ natureconservation.6.5205 Abstract Currently, we are experiencing biodiversity loss on different spatial scales. One of the best studied taxo- nomic groups in decline is the butterflies. Here, we review evidence for such declines using five systematic studies from southern Sweden that compare old butterfly surveys with the current situation. Additionally, we provide data on butterfly and burnet moth extinctions in the region’s counties. In some local areas, half of the butterfly fauna has been lost during the last 60–100 years. -

Effect of Different Mowing Regimes on Butterflies and Diurnal Moths on Road Verges A
Animal Biodiversity and Conservation 29.2 (2006) 133 Effect of different mowing regimes on butterflies and diurnal moths on road verges A. Valtonen, K. Saarinen & J. Jantunen Valtonen, A., Saarinen, K. & Jantunen, J., 2006. Effect of different mowing regimes on butterflies and diurnal moths on road verges. Animal Biodiversity and Conservation, 29.2: 133–148. Abstract Effect of different mowing regimes on butterflies and diurnal moths on road verges.— In northern and central Europe road verges offer alternative habitats for declining plant and invertebrate species of semi– natural grasslands. The quality of road verges as habitats depends on several factors, of which the mowing regime is one of the easiest to modify. In this study we compared the Lepidoptera communities on road verges that underwent three different mowing regimes regarding the timing and intensity of mowing; mowing in mid–summer, mowing in late summer, and partial mowing (a narrow strip next to the road). A total of 12,174 individuals and 107 species of Lepidoptera were recorded. The mid–summer mown verges had lower species richness and abundance of butterflies and lower species richness and diversity of diurnal moths compared to the late summer and partially mown verges. By delaying the annual mowing until late summer or promoting mosaic–like mowing regimes, such as partial mowing, the quality of road verges as habitats for butterflies and diurnal moths can be improved. Key words: Mowing management, Road verge, Butterfly, Diurnal moth, Alternative habitat, Mowing intensity. Resumen Efecto de los distintos regímenes de siega de los márgenes de las carreteras sobre las polillas diurnas y las mariposas.— En Europa central y septentrional los márgenes de las carreteras constituyen hábitats alternativos para especies de invertebrados y plantas de los prados semi–naturales cuyas poblaciones se están reduciendo. -
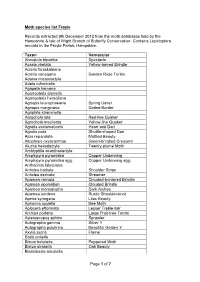
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Check List of Noctuid Moths (Lepidoptera: Noctuidae And
Бiологiчний вiсник МДПУ імені Богдана Хмельницького 6 (2), стор. 87–97, 2016 Biological Bulletin of Bogdan Chmelnitskiy Melitopol State Pedagogical University, 6 (2), pp. 87–97, 2016 ARTICLE UDC 595.786 CHECK LIST OF NOCTUID MOTHS (LEPIDOPTERA: NOCTUIDAE AND EREBIDAE EXCLUDING LYMANTRIINAE AND ARCTIINAE) FROM THE SAUR MOUNTAINS (EAST KAZAKHSTAN AND NORTH-EAST CHINA) A.V. Volynkin1, 2, S.V. Titov3, M. Černila4 1 Altai State University, South Siberian Botanical Garden, Lenina pr. 61, Barnaul, 656049, Russia. E-mail: [email protected] 2 Tomsk State University, Laboratory of Biodiversity and Ecology, Lenina pr. 36, 634050, Tomsk, Russia 3 The Research Centre for Environmental ‘Monitoring’, S. Toraighyrov Pavlodar State University, Lomova str. 64, KZ-140008, Pavlodar, Kazakhstan. E-mail: [email protected] 4 The Slovenian Museum of Natural History, Prešernova 20, SI-1001, Ljubljana, Slovenia. E-mail: [email protected] The paper contains data on the fauna of the Lepidoptera families Erebidae (excluding subfamilies Lymantriinae and Arctiinae) and Noctuidae of the Saur Mountains (East Kazakhstan). The check list includes 216 species. The map of collecting localities is presented. Key words: Lepidoptera, Noctuidae, Erebidae, Asia, Kazakhstan, Saur, fauna. INTRODUCTION The fauna of noctuoid moths (the families Erebidae and Noctuidae) of Kazakhstan is still poorly studied. Only the fauna of West Kazakhstan has been studied satisfactorily (Gorbunov 2011). On the faunas of other parts of the country, only fragmentary data are published (Lederer, 1853; 1855; Aibasov & Zhdanko 1982; Hacker & Peks 1990; Lehmann et al. 1998; Benedek & Bálint 2009; 2013; Korb 2013). In contrast to the West Kazakhstan, the fauna of noctuid moths of East Kazakhstan was studied inadequately. -

The Pyrenees
The Pyrenees A Greentours Holiday for the Alpine Garden Society 10th to 23rd June 2011 Led by Paul Cardy Trip Report and Systematic Lists by Paul Cardy Day 1 Friday 10 th June Arrival and Transfer to Formigueres Having driven from the south western Alps and reached Carcassonne the previous evening, I continued to Toulouse to meet the group at the airport. I was unexpectedly delayed by French customs who stopped me at the toll booth entering the city. There followed a lengthy questioning, as I had to unpack the contents of my suspiciously empty Italian mini-bus and show them my two large boxes of books, suitcase full of clothes, picnic supplies, etc., to convince them my purpose was a botanical tour to the Pyrenees. Now a little late I arrived breathlessly at Toulouse airport and rushed to the gate to meet Margaret, and the New Zealand contingent of Chris, Monica, Archie and Lynsie, hurriedly explaining the delay. Anyway we were soon back on the motorway and heading south towards Foix. White Storks in a field on route was a surprise. We made a picnic stop at a functional aire where there were tables, and a selection of weedy plants. Black Kite soared overhead. Once past Foix and Ax-les- Thermes the scenery became ever more interesting as we wound our way up to a misty Col de Puymorens. There a short stop yielded Pulsatilla vernalis in fruit and Trumpet Gentians. Roadside cliffs had Rock Soapwort, Saxifraga paniculata , and Elder-flowered Orchids became numerous. Now in the Parc Naturel Régional des Pyrénées Catalanes, a fascinating route down into the valley took us through Saillagouse and Mont-Louis before heading up a minor road to the village of Formigueres, our base for the first three nights.